Antioxidative and Antianaemic Potentials of Aqueous Leaf Extract of Lowveld Bead String (Alchornea laxiflora) in Phenylhydrazine-induced Anaemic Wistar Rats and Its Phytochemical Constituents
Keywords:
Oxidation, Blood, Extract, Alchornea laxiflora, ROS, phytochemicalsAbstract
Background: Anaemia, a public health concern, is characterized by a decrease in red blood cell count, hemoglobin, and packed cell volume. Phenylhydrazine, a toxic chemical, is commonly used to induce anaemia in experimental models. This study investigated the antioxidative and antianaemic effects of aqueous leaf extract of Alchornea laxiflora in phenylhydrazine-induced anaemic wistar rats.
Method: Qualitative phytochemical screening and HPLC analysis of the extract were carried out. Thirty female wistar rats weighing between 166-169 g were randomly divided into six (6) groups of five animals each. Groups 1 and 2 served as negative and positive control respectively and treated with 1 mL/kg body weight of distilled water only. Groups 2 to 6 were induced with anaemia by intraperitoneal injection of 36 µL/kg body weight of phenylhydrazine (PHZ). Groups 3 to 5 were treated with 500, 1000 and 1500 mg/kg body weight of aqueous leaf extract of A. laxiflora respectively while group 6 was treated with 2 mg/kg body weight of folic acid. The experiment lasted for 14 days after which the rats were sacrificed and their blood collected for hematological parameters. Antioxidative effects of the extract was also investigated.
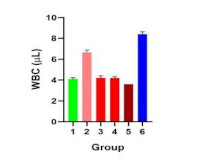
Results: The results showed that the aqueous leaf extract of Alchornea laxiflora is rich in phytochemicals. The extract demonstrated antioxidative properties, as evidenced in its reductive effects on oxidative stress. It also revealed antianaemic effect where it significantly improved hematological parameters such as packed cell volume (PCV), haemoglobin (HB), mean corpuscular haemoglobin concentration (MCHC), mean corpuscular volume (MCV), and red blood cell (RBC).
Conclusion: This study confirmed the presence of various phytochemicals in the aqueous leaf extract of Alchornea laxiflora. The extract demonstrated antioxidative and antianaemic properties. The extract's protective effects against anaemia are likely attributed to its phytochemical constituents.
References
1. Gabriele P, Natasha I, Mariapaola C, Giovanni P, Federica M, Vincenzo A, Francesco S, Domenica A, Alessandra B. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Medicine and Cellular Longevity. 2017;8416763. doi: 10.1155/2017/8416763
2. Chaparro CM, Suchdev PS. An a emi a Epidemiology, Pathophysiology, and Etiology in Low-And Middle-Income Countries. Annals of the New York Academy of Sciences, 2019; 1450, 15-31. https://doi.org/10.1111/nyas.14092.
3. Shubham K, Anukiruthika T, Dutta S, Kashyap AV, Moses JA, Anandharamakrishnan C. Iron Deficiency Anemia: AComprehensive Review on Iron Absorption, Bioavailability and Emerging Food Fortification Approaches. Trends in Food Science and Technology, 2020; 99, 58-75.
4. Orlov D, Karkouti K. The pathophysiology and consequences of red blood cell storage. Anaesthesia, 2015;70, 29-e12.
5. Buttarello M. Laboratory diagnosis of anaemia: are the old and new red cell parameters useful in classification and treatment. International Journal of Laboratory Hematology,2016; 38, 123-132.
6. Pasricha, SR, Tye-Din J, Muckenthaler MU, Swinkels DW. Iron Deficiency, a prevailing effect of anaemia. 2021;397(10270), 233-248.
7. Agbo MO, Okoye FB, Ebi GC, Osadebe PO. Alchornea floribunda; (Müll. Arg.) - a review of its phytochemistry and biological activities. 2020;19, 1113–1120. doi:10.4314/tjpr.v19i5.30
8. Yong-Chang S, Liang-Jun D. Evergreen broadleaved forest of East Asia. Vegetation structure and function at multiple spatial, temporal and conceptual scales, 2016;101-128.
9. Siddiqui AA, Siddiqui SA, Ahmad S, Siddiqui S, Ahsan I, Sahu K. “Diabetes: mechanism, pathophysiology and management- A review”. International Journal of Drug Development and Research 2013;5(2):1-23.
10. Sofowora A. Medicinal plants in Africa. 2nd Ed. Sunshine House, Ibadan, Nigeria: Spectrum Books Ltd; 1993. Screening plants for bioactive agents; Pp. 134-156.
11. Ajuru MG, Williams LF, Ajuru G. Qualitative and quantitative phytochemical screening of some plants used in ethnomedicine in the Niger Delta region of Nigeria. Journal of Food and Nutrition Sciences, 2017;5(5), 198-205.
12. Egbuna, C., Ifemeje, J. C., Maduako, M. C., Tijjani, H., Udedi, S. C., Nwaka, A. C., and Ifemeje, M. O. Phytochemical test methods: qualitative, quantitative and proximate analysis. In Phytochemistry 2018;(pp. 381-426).
13. Niaz M, Abrar H, Ashfaq S, Khan N, Awais M, Baseerat N, Ullah K. Qualitative phytochemical analysis and in vitro antibacterial activity of Punica Granatum. Phytopharmacology Research Journal, 2024;3(1), 31-38.
14. Talukdar A, Chaudhary B. Phytochemical Screening of ethanolic extracts of Rubia Cordiofolia. 2010;1(4): 530-536.
15. Madike LN, Takaidza S, Pillay M. Preliminary phytochemical screening of crude extracts from the leaves, stems, and roots of Tulbaghia violacea. International Journal of Pharmacognosy and Phytochemical Research, 2017;9(10), 1300- 1308.
16. Dulo B, Phan K, Githaiga J, Raes K, De Meester S. Natural quinone dyes: A review on structure, extraction techniques, analysis and application potential. Waste and Biomass Valorization, 2021;12(12), 6339-6374.
17. Adeniyi OA. Isolation and characterisation of quercitrin, a potent anti-sickle cell anaemia agent from the Nigerian shrub, Alchornea Spp (Doctoral dissertation, Aberystwyth University) 2020.
18. Itano HA, Hosokawa K, Hirota K. British journal of haematology 1976;32(1), 99-104
19. Magnani L, Gaydou M, Jean CH. Spectrophotometric measurement of antioxidant properties of flavone and flavonol against
superioxide action, 2000;411 (1-2) 1: 209. 206.
20. Hadwan MH, Hussein MJ, Mohammed RM, Hadwan AM, Saad Al-Kawaz H, Al-Obaidy SS, Al Talebi ZA. An improved method for measuring catalase activity in biological samples. Biology Methods and Protocols, 2024;9(1), bpae015.
21. George-Opuda MI, Adegoke OA, Odeghe BO, Awopeju AT, Okeahialam NM. Assessment of liver antioxidant profile in plasmodium berghei infected mice treated with curative ethanol leaf extract of Musa paradisiaca. Ethiopian Journal of Health Sciences, 2023;33(5), 761-768.
22. Akerboom TP, Seis H. Assay of glutathione, glutathione disulfide, and glutathione mixed disulfides in biological samples. 1981;77:373-382
23. Ajani EO, Bamisaye FA, Amusa TO, Atolani O, Kola-Mustapha AT, Njinga NS, Quadri LA, Bakare-Odunola MT, Oladiji AT. Roselle Hibiscus Sabdarrifa Calyces Extracts Modulates Cardiovascular Disease Risk and Kidney Dysfunctions in Diabetic Rats.
https://doi.org/10.51470/PLANTARCHIVES.2021.v21.S1.212
24. Hoffman R, Benz J, Shattil SJ, Furie B, Silberstein LE. Hematology: Basic Principles and Practice, 2018 (7th ed.).
25. Akinpelu DA, Abioye EO, Aiyegoro OA, Akinpelu OF, Okoh AI. Evaluation of antibacterial and antifungal properties of Alchornea laxiflora (Benth.) Pax. & Hoffman. Evidence‐Based Complementary and Alternative Medicine, 2015(1), 684839.
26. Jain NK, Tailang M, Kumar S, Chandrasekaran B, Alghazwani Y, Chandramoorthy HC, Chidambaram K.. Appraising the therapeutical potentials of Alchornea laxiflora (Benth.) Pax & K. Hoffm., an underexplored medicinal herb: A systematic review. Frontiers in Pharmacology, 2022;13, 958453.
27. Lawal B, Shittu OK, Oibiokpa FI, Berinyuy EB, Mohammed H. African natural products with potential antioxidants and hepatoprotectives properties: a review. Clinical Phytoscience, 2017;2, 1-66.
28. Ahmadinejad F, Geir Møller S, HashemzadehChaleshtori M, Bidkhori G, and Jami M.S. Molecular mechanisms behind free radical scavengers function against oxidative stress. Antioxidants, 2017;6(3), 51.
29. Araoye MO, Abiodun OA, Ogunlade B. Antioxidant activity of Alchornea laxiflora (Benth) leaf extract. Journal of Applied Pharmaceutical Science. 2017;7(10):223-229.
30. Zhang Z, Han X, Liu Z, Jiang X, Sun M, Liu Q. ERNIE: Enhanced language representation with informative entities. arXivpreprint
arXiv:2019;1905.07129.
31. Chukwujekwu JC, Coker HA, Olayide OA. Cardioprotective effects of Alchornea laxiflora in experimental models of cardiac injury. Journal of Medicinal Plants Research, 2020;6(5), 771-777.
32. Yazdanparast R, Bahramikia S, Ardestani A. Nasturtium officinale reduces oxidative stress and enhances antioxidant capacity in hypercholesterolaemic rats. Chemico-Biological Interactions, 2008;172(3), 176-184.
33. Gwozdzinski K, Pieniazek A, Gwozdzinski L. Reactive oxygen species and their involvement in red blood cell damage in chronic kidney disease. Oxidative Medicine and Cellular Longevity, 2021(1), 6639199.
34. Orrico F, Laurance S, Lopez AC, Lefevre SD, Thomson L, Möller MN, Ostuni MA. Oxidative Stress in Healthy and Pathological Red Blood Cells. Biomolecules.2023;13(8):1262. doi: 10.3390/biom13081262
35. Imam MU, Zhang S, Ma J, Wang H, Wang F. Antioxidants mediate both iron homeostasis and oxidative stress. Nutrients, 2017;9(7), 671.
36. Onyeabo C, Achi NK, Ekeleme-Egedigwe CA, Ebere CU, Okoro CK. Haematological and biochemical studies on Justicia carnea leaves extract in phenylhydrazine induced-anemia in wistar rats. Acta Scientiarum Polonorum Technologia Alimentaria,2017; 16(2), 217-230.
37. Salvagno GL, Sanchis-Gomar F, Picanza A, Lippi G. Red blood cell distribution width: a simple parameter with multiple clinical applications. Critical Reviews in Clinical Laboratory Sciences, 2015;52(2), 86-105.
38. Chauhan SP, Sheth NR, Suhagia BN. Hematinic effect of fruits of opuntia elatior Mill. on phenylhydrazine-induced anaemia in rats. Ayu 2021;36(2):208.
Downloads
Views | PDF Downloads:
391
/ 140
Published
How to Cite
Issue
Section
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.


