Phytochemical Analysis of Gas chromatography mass spectrometry (GC-MS) and Hepato-renal protective effects of <I>Terminalia catappa</I> leaf and seed extracts in BPH-induced prostate in rat's model
DOI:
https://doi.org/10.51412/psnnjp.2025.11Keywords:
Terminalia Catappa, phytochemical analysis, GC MS, BPHAbstract
Background: Benign prostatic hyperplasia (BPH) is a prevalent urological condition. The medicinal plant Terminalia catappa has been traditionally utilized for treating diverse ailments. This study explores the phytochemical profile via GC-MS analysis and assesses the hepatorenal protective properties of Terminalia catappa leaf and seed extracts in a rat model with BPH induction.
Methods: BPH was induced in rats using testosterone propionate, followed by oral administration of Terminalia catappa leaf and seed extracts (250 and 500 mg/kg) for 28 days. Liver and kidney function were evaluated through enzyme and biomarker assessments. Additionally, histopathological analyses of hepatic and renal tissues were performed.
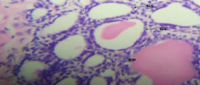
Results: GC-MS profiling revealed various bioactive compounds, including flavonoids, alkaloids, saponins, tannins, steroids, and glycosides. Administration of Terminalia catappa extracts led to significant reductions in prostate weight and epithelial thickness while improving liver enzyme and kidney function markers (ALT, ALP, AST, creatinine, and urea) compared to the control group. Histopathological findings indicated enhanced prostate and liver tissue integrity.
Conclusion: This study highlights the phytochemical composition and the hepatorenal protective potential of T. catappa leaf and seed extracts in a rat model of BPH. The findings suggest that these extracts may serve as a promising therapeutic approach for managing BPH and associated hepatorenal dysfunction.
References
Nickel, C.J. (2012). Lower urinary tract symptoms associated with prostatitis Cancer Urological Association Journal, 6 (5):
S133–S135. https://doi.org/ 10.5489/cuaj.12201
Chughtai et al. (2011). The role of inflammation in benign prostatic hyperplasia. Current Urology Reports, 12(4), 266-273, https://doi.org/10.1007/s11934-011-0191-3
Kapoor, A. (2012). Benign prostatic hyperplasia (BPH) Management in the primary care setting. Cancer Journal of Urology, 19:10-17, Corpus ID 35332548
Kim, D., Jeong, S. W. and Lee, C. Y. (2003). Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chemistry, 81(3): 321-326. https://doi.org/10.1016/S0308-8146(02)00423-5
Panka, O. and Robert, E. P. (2008). West Indian almond terminalia catappa L. combretaceal” In: Janick, J and Paull, R. E. (eds) Encyclopedia of fruit and nuts. United Kingdom: Walling ford, pp.273-276.
Steenkamp, V. (2003). Phytomedicines for prostate fitoterepia. Journal of Clinical Diagonistic, 74: 545 - 550.
https://doi.org/10.1016/S0367-326x (03)00155-2
Cavalcante, M.A., Maia, G.A., Figueiredo, R.W. and Teixeira, V. A. M. (1986). Physical agronomical sciences. Science, 17 (1):111-116.
Orquidea, V. S., Natasha, D. L., and Suzana Caetano, S. L. (2016) Chemical, morphological and thermogravimetric of Terminalia Catappa
Linn. Food Science Technology, 1(36): 151-158. https://doi.org/ 10.1590/1678-457X.0090
Teixeira, H. L. (2010). Composição química e perfil de ácidos graxos da castanha do fruto da castanhola (Terminalia catappa Linn) (Master's thesis). Universidade Estadual do Sudoeste da Bahia, Itapetinga.
Lin, C. C., Hsu, Y. F. and Lin, T. C. (1999). Effects of punicaligin and punicalin on carrageenan induced inflammation in rats. The American Journal of Chinese Medicine, 27:371-376. https://doi.org/10.1142/S0192415X99000422
Zhang et al. (2018). Phytochemical analysis of T. catappa leaves and seeds. Journal of Pharmaceutical Sciences, 107(5): 1441-1448.
Singh et al. (2019). Phytochemicals in the prevention and treatment of benign prostatic hyperplasia. Journal of Pharmacy and Pharmacology, 71(8): 1134-1146.
Pandey et al. (2018). GC-MS analysis of phytochemicals in plant extracts. Journal of Pharmaceutical Analysis, 8(2): 69-77.
Lorke, D. (1983). A new approach to practical toxicity testing. Archives of Toxicology, 54:275-287.
Harborne, J. B. (1998). Phytochemical methods: A guide to modern techniques of plant analysis 3rd ed., London: Chapman and hall, Pp. 302.
Salehi-Surmahgi, M. H., Aynehchi, Y., Amion, G. H. and Mahhmoodi, Z. (1992). Survey of Iranian plants for saponins, alkaloids, flavoniods and tannins. Iv. Journal of Urology, 2:1-11. https://doi.org/10.3109/13880208509070686
Segelman, A. B., Farnsworth, N. R. and Quimby, M. D. (1969). False negative saponins test results induced by the presence of tannins. Journal of Urology, 32:52-58.
Kapoor, L. D., Sing, A., Kapoor, S. L. and Svivastava, S. N. (1969). Survey of indian medicinal plants for saponins, alkaloids and flavonoids. Journal of Therapeutic Medicine, 32:297-302.
Kokate, C. K. Purohit, A. P. and Gokhale, B. (2001). Carbohydrate and derived products, drugs containing glycosides, drugs containing
tannins, lipids and proteins alkaloids. Text Book of Pharmacognosy, 7th ed.
Kumar, G., Banu, G.S., Murugesan, A.G. and Pandian, M.R. (2012). Hypoglycaemic effect of Helicteres isora bark extract in rats. Journal
Ethnopharmacology, 107:304-307
Reitman, S. and Frankel, S. (1957). A colourimetric method for htedetermination of serum glutamine oxaloacetic acid and glutamine pyruvic acid and serum transaminases. American Journal of Clinical Pathology, 28:56-63.
Tobacco, A. (1979). Expert panel on enzyme of the IFCC. Clinical Chemistry, 25(2):336.
Allen, L. C. (1982). Expert panel on enzyme of the IFCC. Clinical chemistry, 28(3):555-559.
Kim, E. H., Larson, J. A. and Andriole, G. L. (2018). “Management of benign prostatic hyperplasia” Annual Review of Medicine, 67:137- 51. https://doi.org/10.1146/annurev-med-063014-123902.
Lordach, A., Culea, M., Gherman, C. and Cozar, O. (2009). Characterization of some plant extracts by GC-MS. Nuclear Inst. and methods in physical research B. Journal of Urological, 34: 338-342. https://doi.org/10.1016/j.nimb.2008.10.021
Pallado, P., Tassinato, G., D'Alpaos, M. and Traldi, P. (1997). Effects of Terminalia catappa on azoxymethane induced colon carcinogenesis in male rats. Rapid Community Mass Spectrum, 11: 1335-1345.
Ratnasooriya, W. D. and Dharmasivi, M. G. (2000). Effects of Terminalia catappa seeds on seual behavior and fertility of male rats. Asian
Journal of Andrology, 2:213-226.
Sosa, A. A., bagi, S. H. and hameed, I. H. (2016). Analysis of bioactive chemical compounds of euphorbia lathyrus using gas chromatography-Mass spectrometry and fouriev-transform infrared spectroscopy. International Journal of Pharmacognosy and Phytochemical research, 8 (5): 109 - 126. https://doi.org/10.5897/JPP2015.0371
Alon, T. and Amirav, A. (2006). Isotope abundance analysis methods and software for improved sample identification with supersonic
gas chromatography/mass spectrometry. Rapid communication Mass Spectron, 20:2579-2588. https://doi.org/10.1002/rcm.2637
Altameme, H. J., Hadi, M. Y. and Hammed, I. H. (2015). Phytochemical analysis of urtica dioica leaves by fourier-transform infraved spectroscopy and gas chromatography mass spectrometry. Journal of Pharmacognosy and Phytotherapy, 7 (7): 238 - 252.
https://doi.org/10.5897/JPP2015.0361.
Mohamed, D. A., Rashed, M. M., Shallan, M., Fouda, K. and Hanna, L. M. (2016). Amehoration of being prostate hyperplasia in rats through plant foods. International Journal Pharmacology and Phytochemical Research, 8(12): 2063-2070.
Mohale, D.S., Dewani, A.N. and Khadse, C.D. (2008). Antihyperlipidemic activity of isolated constituents from Lagenaria siceraria in albino rats. International Journal of Green Pharmaceutical , 2: 104 - 107, https://doi.org/10.22377/ijgp.v2i2.39.
Jing, G.A.O., Xin-Hui, T., Li-Zhi, X., Yi-Mei, F. and Xiao-Ning, Z. (2004). Biochemistry and physiology. Acta Biochi Biophy Sinica, 36:767-772.
Mcvary, K. T. (2006). BPH: Epidemiology and comorbidities. American Journal of Management Care, 125(5): 122-128.
Wu, S. L. and Li, N. C. (2006). Natural history of benign prostate hyperplasia. Clinical Medical Journal England, 119 (24): 2085-2089.
https://doi.org/10.1111/j.1464-410X.2006.06097.x
Faubert, P. F. and Porush, J. G. (1998). Renal disease is the elderly. New York: Marcel Dekker. p.34
Emeje, I. P., Ukibe, N. R., Onyenekwe, C. C. and Nnamah, N. K. (2017). Assessment of serum prostate specific antigen, some renal induces and uric and levels in subjects with beingn prostatic hyperplasia at Lokoja, Nigeria. Journal Bioanalaytical Biomedicine, 9:256-262,
https://doi.org/10.4172/1948-593x.1000189
Kim, E. H., Larson, J. A. and Andriole, G. L. (2016). “Management of benign prostatic hyperplasia” Annual Review of Medicine, 67:137- 51,https://doi.org/10.1146/annurev-med-063014-123902
Downloads
Views | PDF Downloads:
539
/ 184
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 The Nigerian Journal of Pharmacy

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.


