High-Performance Liquid Chromatographic Determination of Folic Acid Content in Pharmaceutical Formulations marketed in Southwest Nigeria.
https://doi.org/10.51412/psnnjp.2023.22
Keywords:
Folic acid, physicochemical properties, assay of content, labelled claim, RP-HPLC, validationAbstract
Background Folic acid is presented as tablets and syrups or incorporated into food, drinks, and beverages frequently for fortification as a remedy to folic acid deficiency which may cause severe health consequences. Consequently, the analysis of folate in pharmaceutical preparations by quantitative methods like high-performance liquid chromatographic (HPLC) in this study cannot be overemphasized
Methods: The parameters investigated included weight uniformity, hardness, friability, and disintegration rate. A validated, reversed-phase HPLC (RP-HPLC) method ran with trifluoroacetic acid and acetonitrile (90:10) as mobile phase, on a C18 column, at 40°C, was used to assay the content. The method employed a flow rate of 1.0 mL/min and a wavelength of 290 nm. The injection volume was 10 µL
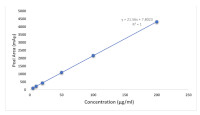
Results: The weight of the tablets ranged between 9.86±0.01-32.30±0.01 mg, hardness was 7.52±0.80-15.48±1.53 kg/cm3, and tablets' friability was less than 1%. All the tablets passed the disintegration test for uncoated tablets except FAJ. Folic acid was eluted at 2.616 min and the RPHPLC method displayed good linearity over the concentration range of 5-200 ppm, with a correlation coefficient (r2) of 0.9998. The relative standard deviation (% RSD) for precision was 0.2%, while the LOD and LOQ values were 1.3191 and 4.3969 µg/ml respectively. The % RSD for reproducibility was < 5% in the assay of folic acid tablet samples. Only 50% of the brands passed the assay of content i.e., fell within the specified range of not less than 90 % and not more than 110 % according to reference specification
Conclusion: None of the brands of folic acid tablets evaluated complied with all the specifications for each test carried out whether compendia or non-compendia test. Hence, stringent quality control including adequate storage control should be applied for the release of such vitamin preparations.
References
Combs GF (2008) The vitamins: fundamental aspects in nutrition and health. Elsevier Academic Press:Amsterdam.
Wang F, Cao M, Wang N, Muhammad N, Wu S, Zhu Y (2018) Analytical methods simple coupled ultrahigh performance liquid chromatography and ion chromatography technique for simultaneous determination of folic acid and inorganic anions in folic acid tablets. Food Chemistry 239: 62-67
Bailey RL, West KP Jr and Black RE (2015) The epidemiology of global micronutrient deficiencies. Annals of Nutrition and Metabolism 66 (Suppl 2): 22–33.
FAO, IFAD, UNICEF, WFP, WHO (2018) The state of food security and nutrition in the world. Rome, Italy: Food and Agricultural Organization of the UN. A v a i l a b l e f r o m : www.fao.org/3/i9553en/i9553en.pdf. Accessed April 20, 2022
Darnton-Hill I (2017) Prevalence causes and consequences of micronutrient deficiencies. Chapter 2, In Mannar V, Hurrell R, editors. Food fortification in a globalized world London, UK:Academic Press/.
Djukic A (2007) Folate-responsive neurologic
diseases. Paediatric Neurology 37: 387-397. https://doi. 10.1016/j.pediatrneurol.2007.09.001. AccessedApril 20, 2022
Hodgetts V, Morris R, FrancisA, Gardosi J, Ismail K (2015) Effectiveness of folic acid supplementation in pregnancy on reducing the risk of small- forgestational-age neonates: a population study, systematic review, and meta-analysis. BJOG: International Journal of Gynaecology and Obstetrics 122 (4): 478–490.
Christensen B, Arbour L, Tran P, Leclerc D, Sabbaghian N, PlaƩ R, Gilfix B M, RosenblaƩ DS, Gravel RA, Forbes P, Rozen R (1999) Genetic polymorphisms in methylenetetrahydrofolate reductase and methionine synthase, folate levels in red blood cells, and risk of neural tube defects. American Journal of Medical Genetics 84 (2): 151-157.
EFSA/NDA Panel (European Food Safety Authority (EFSA) (2014) Panel on Dietetic Products, Nutrition andAllergies (NDA)). 59. Scientific Opinion on Dietary Reference Values for Folate. EFSA J. 12(11), 3893. https://doi: 10.2903/j.efsa.2014.3893 Available from: www.efsa.europa.eu/efsajournal. AccessedApril 06, 2022
Arnarson A (2022) Folic Acid vs. Folate—What's t h e D i ff e r e n c e ? A v a i l a b l e o n hƩps://www.healthline.com/nutriƟon/folicacid-vs-folate. Accessed March 24, 2022.
Lykstad J and Sharma S (2019) Biochemistry, Water Soluble Vitamins. In: StatPearls. Treasure Island (FL): StatPearls Publishing. Available from: hƩps://www.ncbi.nlm.nih.gov/books/NBK5385 10/. AccessedApril 20, 2022
Duthie SJ (1999) Folic acid deficiency and cancer: mechanisms of DNA instability. British Medical B u l l e t i n 5 5 ( 3 ) : 5 7 8 - 5 9 2 . https://doi:10.1258/0007142991902646. PMID: 10746348.
Micronutrient Information Centre. Folate | Linus Pauling InsƟtute | Oregon State University. A v a i l a b l e f r o m : hƩps://lpi.oregonstate.edu/mic/vitamins/folate .Assessed on 16 May 2022. Remington: The Science and PracƟce of Pharmacy
. Remington's Pharmaceutical Sciences by Joseph P. Remington, Mack Publishing Company, 18th Edition. 441 – 443.
Nagaraja P, Vasantha RA and Yathirajan HS (2002) Spectrophotometric determination of folic acid in pharmaceutical preparations by coupling reactions with aminodibenzyl or 3-aminophenol or sodium molybdate-pyrocatechol. Analytical Biochemistry 307: 316-337.
Cimpoiu C and Hosu A (2007) Thin layer chromatography for the analysis of vitamins and their derivatives. Journal of Liquid Chromatography and Related Technologies 30 (5-7): 701-728.
Nelson BC, Sharpless KE and Sander LC (2006) Quantitative determination of folic acid in multivitamin/multielement tablets using liquid chromatography/tandem mass spectrometry. Journal of Chromatography A. 1135(2): 203-211. https://doi: 10.1016/j.chroma.2006.09.040.
Zheng X, Jiang L, Zhao L, Zhang Q, Ding L (2015) Simultaneous quantitation of folic acid and 5- methyltetrahydrofolic acid in human plasma by H P L C – M S / M S a n d i t s a p p l i c a t i o n t o a pharmacokinetic study. Journal of Pharmaceutical Analysis 5(4): 269-275
ANVISA (2010) Brazilian pharmacopoeia. 5th edn. ANVISA, Brasília. 60-114. Published by the Brazilian Health SurveillanceAgency.Available from: www.anvisa.gov.br.AccessedApril 06, 2022
Shaikh KA and Patil SD (2010) Sensitive and selective method for the analysis of menthol from pharmaceutical products by RP-HPLC with refractive index detector. Journal of Pharmacy and Bioallied Sciences 2(4): 360-364
Bouvier ESP and Koza SM (2014) Advances in size-exclusion separations of proteins and polymers by UHPLC. Trends in Analytical Chemistry 63: 85- 94.
The United States Pharmacopeia (2002) 25/The National Formulary 20; The United States Pharmacopeial Convention, Inc.: Rockville, MD, 1958-1959.
Kumar TNV, Vidyadhara S, Narkhede NK, Silpa YS, Lakshmi MR (2016) Method development, validation, and stability studies of teneligliptin by RPHPLC and identification of degradation products by UPLC tandem mass spectroscopy. Journal of Analytical Science and Technology 7: 27. https://doi: 10.1186/s40543-016-0099-0.AccessedApril 23, 2022
Microsoft Corporation (2016) Microsoft Excel. R e t r i e v e d f r o m hƩps://office.microsoŌ.com/excel. Accessed April 02, 2022
British Pharmacopoeia Commission. (2009) British Pharmacopoeia Vol. III. London: The Stationery Office Limited. 6578-6585.
Alderborn G (2013) Tablets and compaction. In: Aulton ME, Taylor KMG, editors. Pharmaceutics. The design and manufacture of medicines. 4th edn. Edinburgh: Churchill Livingstone. 505-549.
Devnath GP, Kumaran S, Rajiv R, Shaha KK, Nagaraj A (2017) Fatal folic acid toxicity in humans. Journal of Forensic Science 62 (6): 1668-1670. https://doi: 10.1111/1556-4029. 13489. Epub 2017. AccessedApril 02, 2022
USP (2018) The United States Pharmacopeia (USP 41) The National Formulary (NF 36) 2017. The United States Pharmacopeial Convention, 12601, Rockville, MD 20852.
Sheorey SD, Hinge MA, Sengupta R, Menon BV (2012) Pharmaceutical equivalence between different brands of metformin hydrochloride tablets. Journal of Pharmaceutical Research 5(6): 3456-3459.
Rana AS and Kumar SLH (2013) Manufacturing defects of tablets - a review. Journal of Drug Delivery and Therapeutics 3(6): 200-206. Available from hƩp://jddtonline.info.AccessedApril 10, 2022
FAO/WHO. FAO/WHO Expert Consultation (1988) Requirements of Vitamin A, Iron, Folate and Vitamin B12. pp. 51-61. Rome, FAO.
Deopa B., Parakh M., Dara P., Payal V., Chordiya K., Panday A., Singh S., Parashar D. (2018). Effect of folic acid supplementation on seizure control in epileptic children receiving long term antiepileptic therapy. Indian Journal of Paediatrics 85 (7): 493-497. https://doi: 10.1007/s12098-018-2608-1. Epub 2018.
Martins-Júnior HA, Wang AY, Alabourda J, Pires MAF, Vegac OB, Lebre DT (2008) A validated method to quantify folic acid in Wheat flour samples using liquid chromatography-Tandem Mass Spectrometry. Journal of the Brazilian Chemical Society 19(5): 971- 977
Obaji SG and Al-Ismail S (2015) Isolated folate deficiency causing profound pancytopenia in pregnancy. The British Medical Journal Case Reports https://doi: 10.1136/bcr-2014-207861
BuƩerworth Jr CE and Tamura T (1989) Folic acid safety and toxicity: a brief review. The American Journal of Clinical Nutrition 50(2): 353-358. https://doi: 10.1093/ajcn/50.2.353
Views | PDF Downloads:
877
/ 330
/ 0
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 The Nigerian Journal of Pharmacy

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.


