Development of a Colorimetric Method for Determination of Piroxicam in Pharmaceutical Formulation Based on Charge Transfer Complex Formation Using Picric Acid
Keywords:
assay, charge transfer complex, NSAID, piroxicam, spectrophotometryAbstract
Background: Piroxicam is a non-steroidal anti-inflammatory drug (NSAID) that is used to manage mild to moderate pain, arthritis, and other inflammatory conditions. There is a dearth of simple, cost-effective, and reliable colorimetric methods for the routine determination of piroxicam in pharmaceutical formulations. The study was aimed at developing a colorimetric method for quantifying piroxicam based on charge-transfer complexation between piroxicam and picric acid.
Methods: The method is based on the formation of a charge-transfer complex between piroxicam an electron donor and picric acid as the electron acceptor. The yellow coloured product formed was quantified at the absorption maximum of 420 nm. The effect of various experimental conditions (reaction time, solvent type and reagent concentration) on complex formation were also investigated. The method was validated as per ICH guidelines. The accuracy was evaluated using recovery studies while precision was evaluated on intra-day and inter-day basis. The limit of detection (LOD), limit of quantification (LOQ) and molar absorptivity were determined and the method was compared with the official titrimetric assay method for the drug.
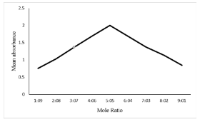
Results: The optimized conditions for complex formation were found to involve the use of Dichloromethane as solvent, a reaction time of 10 minutes and 2.5 ml of 0.001% picric acid solution. 2 Beer's law was obeyed in the concentration range of 20 – 100 μg/ml (r = 0.994). The method showed good precision with inter-day precision in the range of 0.03 – 0.91 % RSD while intra-day precision was from 0.02 – 0.85 % RSD. A comparison of the proposed method with the official method revealed that they produce comparable analytical results.
Conclusion: The developed method was applied successfully to the analysis of piroxicam in capsule formulation with good accuracy and precision and without any interference from the excipients. As such, the newly developed method can serve as a useful technique for the quality assessment of piroxicam.
References
Sadeghi M and Shabani-Nooshabadi M (2021) High sensitive titanium/chitosan-coated nanoporous gold film electrode for electrochemical determination of acetaminophen in the presence of piroxicam. Progress in Organic Coatings, 151: 106100 https://www.sciencedirect.com/science/article/pii/S0300944020313114
Mishra M, Chawla V and Chawla P (2019) Pectin, beta-cyclodextrin, chitosan and albumin based gastroprotective systems for piroxicam maleate: Synthesis, characterization and biological evaluation. International Journal of Biological Macromolecules, 122: 127–36
https://www.sciencedirect.com/science/article/pii/S0141813018350220
Raja MU, Tauchman J, Therrien B, Süss-Fink G, Riedel T and Dyson PJ (2014) Arene ruthenium and pentamethylcyclopentadienyl rhodium and iridium complexes containing N,O-chelating ligands derived from piroxicam: Synthesis, molecular structure and cytotoxicity. Inorganica Chimica Acta, 409: 479–83. https://www.sciencedirect.com/science/article/pii/S0020169313004611
Abdeen A, Aboubakr M, Elgazzar D, Abdo M, Abdelkader A, Ibrahim S and Elkomy A (2019) Rosuvastatin attenuates piroxicam-mediated gastric ulceration and hepato-renal toxicity in rats. Biomedicine and Pharmacotherapy, 110: 8 9 5 – 9 0 5 .
https://www.sciencedirect.com/science/article/pii/S075333221837080X
Samra MM, Hafeez H, Azam M, Imran M and Basra MAR (2023) Bi(III) complexes of piroxicam and meloxicam: Synthesis, characterization, antioxidant, anti-inflammatory and DNA cleavage studies. Journal of Molecular Structure, 1272: 134234.
https://www.sciencedirect.com/science/article/pii/S0022286022018853
Salma H, Melha YM, Sonia L, Hamza H and Salim N (2021) Efficient Prediction of In Vitro Piroxicam Release and Diffusion From Topical Films Based on Biopolymers Using Deep Learning Models and Generative Adversarial Networks. Journal of Pharmaceutical Science, 110 (6): 2531 – 43. https://www.sciencedirect.com/science/article/pii/S0022354921000745
Kim S, Chen J, Cheng T, Gindulyte A, He J, He S, Li Q, Shoemaker BA, Thiessen PA, Yu B, Zaslavsky L, Zhang J, and Bolton EE (2019)
Piroxicam | C15H13N3O4S – PubChem 2019 update: improved access to chemical data. https://pubmed.ncbi.nlm.nih.gov/30371825/
Ong SM, Saeki K, Tanaka Y, Nishimura R and Nakagawa T (2016) Effects of etoposide alone and in combination with piroxicam on canine
osteosarcoma cell lines. The Veterinary Journal, 2 1 8 : 51 – 59 . https://www.sciencedirect.com/science/article/pii/S1090023316301927
Szczęśniak-Sięga BM, Mogilski S, Wiglusz RJ, Janczak J, Maniewska J, Malinka W and Filipek B (2019) Synthesis and pharmacological evaluation of novel arylpiperazine oxicams derivatives as potent analgesics without ulcerogenicity. Bioorganic and Medicinal Chemistry, 27 (8):1619 – 28. https://www.sciencedirect.com/science/article/pii/S0968089618316018
British Pharmacopoeia (2009) Monographs. The British Pharmacopoeia Commission. London: The Pharmaceutical Press, Her Majestys Stationary Office. Volume 1, p 586.
USP (2019) The United State Pharmacopeia. Rockville (MD): The United States Pharmacopeial Convention.
Crecelius A, Clench MR, Richards DS and Parr V (2004) Quantitative determination of Piroxicam by TLC - ALDITOFMS. Journal of
Pharmaceutical and Biomedical Analysis, 35 (1): 31–9.
Li X, Gao Y, Liu J, Zhang G and Zhang T (2018) A rapid analysis of piroxicam in beagle plasma applying evaporation-free liquid-liquid extraction by supercritical fluid chromatography-tandem mass spectrometry. Journal of Chromatography B , 1100 – 1101: 93 – 99
https://www.sciencedirect.com/science/article/pii/S157002321830998X
Kormosh ZA, Hunka IP and Bazel YR (2011) Spectrophotometric determination of piroxicam. Journal of Analytical Chemistry, 66 (4): 378–83.
Basan H, Göğer NG, Ertaş N and Orbey MT (2001) Quantitative determination of piroxicam in a new formulation (piroxicam–β-cyclodextrin) by derivative UV spectrophotometric method and HPLC. Journal of Pharmaceutical and Biomedical Analysis, 26 (2): 171 –16 8.
https://www.sciencedirect.com/science/article/pii/S0731708501003831
Escandar GM, Bystol AJ and Campiglia AD (2002) Spectrofluorimetric method for the determination of piroxicam and pyridoxine. Analytica Chimica Acta, 466 (2): 275–83.
Zhang J, Li Q, Liu Z and Zhao L (2023) Rapid and sensitive determination of Piroxicam by N-doped carbondots prepared by plant soot. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 299: 122833. https://www.sciencedirect.com/science/article/pii/S1386142523005188
Pulgarín JAM, Molina AA and Boras N (2010) Determination of piroxicam in pharmaceutical preparations by continuous - flow
chemiluminescence. Analytical Methods, 2 (1): 76–81.
Dal AG, Oktayer Z, Doğrukol-Ak D. Validated method for the determination of piroxicam by capillary zone electrophoresis and its application to tablets. Journal of Analytical Methods in Chemistry, 2014;2014:352698.
Donato MG, Baeyens W, Van Den Bossche W and Sandra P (1994) The determination of non-s t e r o i d a l a n t i i n fl a m m a t o r y d r u g s i n pharmaceuticals by capillary zone electrophoresis and micella relectro kinetic capillary chromatography. Journal of Pharmaceutical and Biomedical Analysis, 12 (1): 21–6.
De Jager AD, Ellis H, Hundt HKL, Swart KJ and Hundt AF (1999) High-performance liquid chromatographic determination with amperometric detection of piroxicam in human plasma and tissues. Journal of Chromatography B: Biomedical Sciences and Applications, 729 (1–2): 183–9
Dragomiroiu GTAB, Cimpoieşu A, Ginghină O, Baloescu C, Bârcă M, Popa DE, Ciobanu A and Anuţa V (2015) The development and validation of a rapid HPLC method for determination of piroxicam. Farmacia, 63 (1): 123–31
Kong FY, Li RF, Yao L, Wang ZX, Lv WX and Wang W (2019) Pt nanoparticles supported on nitrogen doped reduced graphene oxide-single wall carbon nanotubes as a novel platform for highly sensitive electrochemical sensing of piroxicam. Journal of Electroanalytical
Chemistry, 832: 385 – 91. https://www.sciencedirect.com/science/article/pii/S1572665718307598
Alizadeh N and Keyhanian F (2014) Sensitive and selective spectrophotometric assay of piroxicam in pure form, capsule and human blood serum samples via ion-pair complex formation. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 130: 238–44.
https://www.sciencedirect.com/science/article/pii/S1386142514004855
Assubaie FN (2013) Utilization of charge transfer complex formation for the spectrophotometric determination of piroxicam and tenoxicam. Analytical Chemistry: An Indian Journal, 13 (1): 69–76
Abdulrahman SMA, Devi OZ, Basavaiah K and Vinay KB (2016) Use of picric acid and iodine as electron acceptors for spectrophotometric determination of lansoprazole through a charge-transfer complexation reaction. Journal of Taibah University for Science, 10 (1): 80-91
Chaitanya M, Kumar TH, Koduru S and Kalepu S (2025) Quantification of Aprepitant Via Charge Transfer Complexation Reactions Through Visible Spectrophotometric Methods. Journal of Applied Spectroscopy, 91: 1418 – 1427. https://doi.org/10.1007/s10812-025-01868-3
AOAC. (2005) Official Methods of Analysis. 18th Edition. Washington DC: Association of Official Analytical Chemists.
Adam AMA, Saad HA, Refat MS and Hegab MS (2022) Charge - transfer complexes of antipsychotic drug sulpiride with inorganic and organic acceptors generated through two different approaches: Spectral characterization. Journal of Molecular Liquids, 353: 118819. https://www.sciencedirect.com/science/article/pii/S0167732222003567
Haque SKM, Umar Y, Al-Batty S, Sarief A, Abu-Judeh A, Al-Awwad H and Rahman H (2023) Charge transfer based green spectrophotometric method to determine remogliflozin etabonate applying response surface methodology supported with computational studies in pharmaceutical formulations. Sustainable Chemistry and Pharmacy, 35: 101193
Gowda BG, Seetharamappa J and Melwanki MB (2002) Indirect Spectrophotometric Determination of Propranolol Hydrochloride and Piroxicam in Pure and Pharmaceutical Formulations. Analytical Sciences, 18 (6): 671–4. https://doi.org/10.2116/analsci.18.671
Okeri HA and Anthonia UO (2007) Colorimetric Analysis of Piroxicam. Pakistan Journal of Scientific and Industrial Research, 50 (1): 1–4
Food Drug Administrations (2020) Office of Regulatory Affair, ORA-LAB-Manual Volume II. ORALAB.5.4.5. Methods, Method Verification
and Validation. https://www.fda.gov/media/73920/download
El‐D idamo n y A M and A min A S (2004) Adaptation of a Color Reaction for Spectrophotometric Determination of Diclofenac Sodium and Piroxicam in Pure Form and in Pharmaceutical Formulations. Analytical Letters, 37 (6): 1151–62. https://doi.org/10.1081/AL-120034060
International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (2005) (ICH) Q2(R1) Validation of Analytical Procedures: Text and Methodology.
Amin AS (2002) Spectrophotometric determination of piroxicam and tenoxicam in pharmaceutical formulations using alizarin.
Journal of Pharmaceutical and Biomedical Analysis, 29 (4): 729–36. https://www.sciencedirect.com/science/article/pii/S0731708502000353
Downloads
Views | PDF Downloads:
497
/ 219
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 The Nigerian Journal of Pharmacy

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.


