Evaluation of the quality of frequently prescribed antidiabetic medications in Nigeria.
Keywords:
Glibenclamide, Metformin, Diabetes, Quality assessmentAbstract
Background: Diabetes mellitus is a growing public health concern in Nigeria, and ensuring the quality of antidiabetic medications is crucial for effective management. This study aimed to evaluate the pharmaceutical quality of various marketed brands of metformin and glibenclamide tablets in Nigeria.
Methods: The study assessed the physicochemical properties of seven metformin and six glibenclamide brands, including weight variation, hardness, friability, disintegration time, assay, and dissolution profiles. The results were compared to British Pharmacopoeia (BP) and United States Pharmacopoeia (USP) standards.
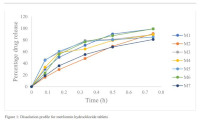
Results: Most brands (~80 %) met the BP/USP specifications for physicochemical parameters. However, dissolution testing revealed variability in release profiles, with only two metformin brands showing similarity to the reference product based on f1 (≤15) and f2 (f2 ≥50) comparison. None of the glibenclamide brands met the similarity criteria.
Conclusion: Continuous post-marketing surveillance and stricter regulatory oversight is recommended to ensure consistent product quality and therapeutic reliability
References
1. Blair M. Diabetes Mellitus Review. Urol Nurs. 2016 Jan-Feb;36(1):27-36.
2. Uloko AE, Musa BM, Ramalan MA, Gezawa ID, Puepet FH, Uloko AT, Borodo MM, Sada KB. Prevalence and Risk Factors for Diabetes Mellitus in Nigeria: A Systematic Review and MetaAnalysis. Diabetes Ther. 2018 Jun;9(3):1307-1316. doi: 10.1007/s13300-018-0441-1
3. Adeloye D, Ige JO, Aderemi AV, Adeleye N, Amoo EO, Auta A, Oni G. Estimating the prevalence, hospitalisation and mortality from type 2 diabetes mellitus in Nigeria: a systematic review and meta-analysis. BMJ Open. 2017 May 11;7(5):e015424. doi: 10.1136/bmjopen-2016-015424.
4. Olamoyegun MA, Alare K, Afolabi SA, Aderinto N, Adeyemi T. A systematic review and metaanalysis of the prevalence and risk factors of type 2 diabetes mellitus in Nigeria. Clin Diabetes Endocrinol. 2024 Dec 6;10(1):43. doi: 10.1186/s40842-024-00209-1.
5. Khunti K, Zaccardi F, Amod A, Aroda VR, Aschner P, Colagiuri S, Mohan V, Chan JCN. Glycaemic control is still central in the hierarchy of priorities in type 2 diabetes management. Diabetologia. 2025 Jan;68(1):17-28. doi: 10.1007/s00125-024-06254-w.
6. Saraswati K, Sichanh C, Newton PN, Caillet C. Quality of medical products for diabetes management: a systematic review. BMJ Glob Health. 2019 Sep 24;4(5):e001636. doi: 10.1136/bmjgh-2019-001636.
7. World Health Organisation. AStudy on the Public Health and Socioeconomic Impact of Substandard and Falsified Medical Products. 2017 Available at: https://www.who.int/publications/i/item/978924 1513432 (Accessed May 6, 2025).
8. Ozawa S, Higgins CR, Yemeke TT, Nwokike JI, Evans L, Hajjou M, Pribluda VS. Importance of medicine quality in achieving universal health coverage. PLoS One. 2020 Jul 9;15(7):e0232966. doi: 10.1371/journal.pone.0232966.
9. Musa GA. Evaluation of The Pharmaceutical Quality of Different Brands of Glibenclamide Tablets Marketed in Kaduna, Nigeria. Nigerian Journal of Pharmaceutical and Biomedical Research, 2019;4(2):192-197.
10. Offu OF. A Systematic Review of the Prevalence and Treatment of Type 2 Diabetes in Nigeria. IDOSR Journal of Biology, Chemistry and Pharmacy, 2019;3(1):124-146.
11. Baker C, Retzik-Stahr C, Singh V, Plomondon R, Anderson V, Rasouli N. Should metformin remain the first-line therapy for treatment of type 2 diabetes? Ther Adv Endocrinol Metab. 2021 Jan 13; 12: 2042018820980225. doi: 10.1177/2042018820980225.
12. Eraga SO, Arhewoh MI, Oruh EP, Iwuagwu MA. A comparative evaluation of the pharmaceutical quality of different brands of metformin hydrochloride tablets available in Abuja, Nigeria. West African Journal of Pharmacy, 2017;28(1):61-71.
13. Fadare J, Olamoyegun M, Gbadegesin BA. Medication adherence and direct treatment cost among diabetes patients attending a tertiary healthcare facility in Ogbomosho, Nigeria. Malawi Med J. 2015 Jun;27(2):65-70. doi: 10.4314/mmj.v27i2.7.
14. Osuafor NG, Ukwe CV, Okonta M. Evaluation of availability, price, and affordability of cardiovascular, diabetes, and global medicines in Abuja , Nigeria. PLoS One . 2021 Aug 12; 16(8): e0255567. doi:10.1371/journal.pone.0255567.
15. Gabel J, Lächele M, Sander K, Gnegel G, SunnyAbarikwu N, Ohazulike RE, Ngene J, Chioke JF, Häfele-Abah C, Heide L. Quality of Essential Medicines from Different Sources in Enugu and Anambra, Nigeria. Am J Trop Med Hyg. 2024 May 14;111(1):179-195. doi: 10.4269/ajtmh.23-0837.
16. Pharmapproach Quality Control Tests for Chewable Tablets, Pharmapproach.com. Pha rmapproa ch Limit ed. Ava il abl e a t:
https://www.pharmapproach.com/qualitycontrol-and-evaluation-parameters-forchewable-tablets/(Accessed: May 6, 2025).
17. Chavan H, Chhabra G, Gujarathi N, Jadhav A.Comparative study of In-process and finished products quality control test for tablet and capsules according to pharmacopoeias. Asian Journal of Pharmaceutical Research and Development, 2018;6(3):60-68.
18. Ibekwe NN, Isaac JA, Ajiboye A, Adigwe OP. Interchangeability for brands of promethazine teoclate (25 mg) tablets available in Abuja metropolis of Nigeria. West African Journal of Pharmacy, 2022;33: 47–52.
19. Osei-Yeboah F, Sun CC. Validation and applications of an expedited tablet friability method. Int J Pharm. 2015 Apr 30;484(1-2):146-55. doi: 10.1016/j.ijpharm.2015.02.061.
20. Isaac JA, Hayatu GI, John JE, Ekere KE, Daburi A, Omachoko SO. Quality assessment of brands of prednisolone (5 Mg) tablets marketed in abuja metropolis of Nigeria. Dissolution Technol, 2021;28(1):24-31.
21. Rahman MM, Jahan FI, Fahim NF, Paul N, Jahan N, Harun-Or-Rashid M. In vitro comparative quality evaluation of leading brands of metronidazole tablets available in Bangladesh. Pharmacology Online,2020;2:63-72.
22. Al-Kubati SS, Ahmed OS, Saif RM. The evaluation of some brands of levocetrizine dihydrochloride film-coated tablets for quality and equivalence sold in Aden, Yemen. Electronic Journal of University of Aden for Basic and Applied Sciences, 2022;3(4):313-321.
23. Alam MN. Pulsatile release drug delivery system based on compression-coated tablets (Doctoral dissertation).
http://dx.doi.org/10.17169/refubium-40005
24. Masih A, Kumar A, Singh S, Tiwari AK. Fast dissolving tablets: a review. International Journal o f Current Pharmaceutical Research, 2017;9(2):8-18.
25. Quodbach J, Kleinebudde P. A critical review on tablet disintegration. Pharm Dev Technol. 2016 Sep; 21(6): 763 - 74. doi:
10.3109/10837450.2015.1045618.
26. Metry M, Shu Y, Abrahamsson B, Cristofoletti R, Dressman JB, Groot DW, Parr A, Langguth P, Shah VP, Tajiri T, Mehta MU, Polli JE. Biowaiver Monographs for Immediate Release Solid Oral Dosage Forms: Metformin Hydrochloride. J Pharm Sci. 2021 Apr;110(4):1513-1526. doi: 10.1016/j.xphs.2021.01.011.
27. Ghori MU, Conway BR. Hydrophilic matrices for oral control drug delivery. American Journal of Pharmacological Sciences, 2015;3(5):103-109.
28. Mardiyanto M, Fithri NA, Amriani A, Herlina H, Sari DP. Formulation and Characterization of Glibenclamide Solid Lipid Submicroparticles Formated by Virgin Coconut Oil and Solid Matrix Surfactant. Science and Technology Indonesia, 2021;6(2):58-66.
29. Leão AD, Oliveira VV, Marinho FA, Wanderley AG, Aguiar JS, Silva TG. Hybrid systems of glibenclamide and layered double hydroxides for solubility enhancement for the treatment of diabetes mellitus II. Applied Clay Science, 2019; 181:105218.
30. Sandri G, Bonferoni MC, Rossi S, Caramella CM, Ferrari F (2018) Effects of particle size, surface nature and crystal type on dissolution rate. Particles and Nanoparticles in Pharmaceutical Products: Design, Manufacturing, Behavior and Performance, 2018:303-328.
31. Center for Drug Evaluation and Research. Bioavailability Studies Submitted in NDAs or INDs – General Considerations. FDA. 2024. Available at: https://www.fda.gov/regulatoryinformation/search-fda-guidance-documents/bioavailability-studies-submittedndas-or-inds-general-considerations (Accessed: 23 May 2025)
Downloads
Views | PDF Downloads:
456
/ 190
Published
How to Cite
Issue
Section
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.


