<i>In Vitro</i>–<i>In Vivo</i> Correlation-Based Evaluation of Bioequivalence in Commercial Paracetamol Brands in Nigeria
Keywords:
paracetamol, bioequivalence, convolution, in vitro-in vivo correlation, AUC, CmaxAbstract
Background: Paracetamol is a widely used over-the-counter analgesic and antipyretic in Nigeria. Ensuring the quality and therapeutic consistency of oral paracetamol products is a critical public health concern. Bioequivalence assessment is essential to confirm that generic drugs match the reference product in terms of rate and extent of systemic drug exposure.
Methodology: This study employed a convolution-based in vitro-in vivo correlation (IVIVC) model to evaluate the bioequivalence of 18 commercial paracetamol tablet brands marketed in Nigeria. The physicochemical properties of the tablets were evaluated, and in vitro dissolution tests were conducted. The IVIVC model was used to predict the pharmacokinetic parameters of the tablets.
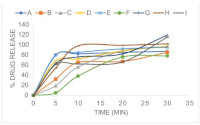
Results: While most products met basic pharmacopeia standards for assay, disintegration and dissolution, none reached full bioequivalence when compared to worldwide regulatory benchmarks for systemic exposure. Although C estimates were acceptable for the majority, all generics had high max AUC prediction errors, ranging up to 51.8 % indicating potential underexposure and treatment
variability.
Conclusions: The findings emphasize the limits of using only in vitro testing to determine therapeutic equivalency, particularly for medicines with fast absorption and broad use, such as paracetamol. Regulatory authorities should consider incorporating IVIVC-based assessments and dissolution similarity indicators into routine post-market surveillance to ensure the quality and efficacy of paracetamol products.
References
1. Offor SJ, Amadi CN, Chijioke-Nwauche I, Manautou JE, Orisakwe OE. Potential deleterious effects of paracetamol dose regime used in Nigeria versus that of the United States of America. Toxicol Rep. 2022 Apr 27;9:1035-1044. doi: 10.1016/j.toxrep.2022.04.025.
2. Adedeji WA, Dairo MD, Nguku PM, Oyemakinde A, Fehintola FA. Pattern and predictors of medication use among adults in southwestern Nigeria: A community-based cross-sectional study. Pha rma col Re s Pe rspe c t. 2023 Feb;11(1):e01017. doi: 10.1002/prp2.1017.
3. Enuagwuna FC, Tobin-West CI, Dappa FA, Bethel TC. Prevalence and Pattern of Analgesic Abuse Among Undergraduate Students of University of Port Harcourt, Rivers State, Nigeria. Niger Med J. 2 0 2 5 A p r 3 ; 6 6 ( 1 ) : 1 4 2 - 1 5 5 . d o i : 10.71480/nmj.v66i1.647
4. U.S. Food and Drug Administration. Overview of in vivo Bioavailability (BA) and Bioequivalence (BE) Studies Supporting NDAs and ANDAs. Available at: https://www.fda.gov/media/166003/download (Accessed: June 30, 2025).
5. Chow SC. Bioavailability and Bioequivalence in Drug Development. Wiley Interdiscip Rev Comput St a t. 2014;6(4):304-312. doi: 10.1002/wics.1310
6. Zaborenko N, Shi Z, Corredor CC, Smith-Goettler BM, Zhang L, Hermans A, Neu CM, Alam MA, Cohen MJ, Lu X, Xiong L, Zacour BM. FirstPrinciples and Empirical Approaches to Predicting In Vitro Dissolution for Pharmaceutical Formulation and Process
Development and for Product Release Testing. AAPS J. 2019 Feb 21; 21 (3): 32. doi: 10.1208/s12248-019-0297-y.
7. Suarez-Sharp S, Cohen M, Kesisoglou F, Abend A, Marroum P, Delvadia P, Kotzagiorgis E, Li M, Nordmark A, Bandi N, Sjögren E, Babiskin A, Heimbach T, Kijima S, Mandula H, Raines K, Seo P, Zhang X. Applications of Clinically Relevant Dissolution Testing: Workshop Summary Report. AAPS J. 2 0 1 8 Au g 2 7 ; 2 0 ( 6 ): 9 3 . d o i: 10.1208/s12248-018-0252-3.
8. Cascone S, Lamberti G, Marra F, Titomanlio G, d'Amore M, Barba AA. Gastrointestinal behavior and ADME phenomena: In vitro simulation. Journal of Drug Delivery Science and Technology, 2016; 35: 272 - 283. https://doi.org/10.1016/j.jddst.2016.08.002
9. Vinarov Z, Abdallah M, Agundez JAG, Allegaert K, Basit AW, Braeckmans M, Ceulemans J, Corsetti M, Griffin BT, Grimm M, Keszthelyi D, Koziolek M, Madla CM, Matthys C, McCoubrey LE, Mitra A, Reppas C, Stappaerts J, Steenackers N, Trevaskis NL, Vanuytsel T, Vertzoni M, Weitschies W, Wilson C, Augustijns P. Impact of gastrointestinal tract variability on oral drug absorption and pharmacokinetics: An UNGAP review. Eur J Pharm Sci. 2021 Jul 1;162:105812. doi: 10.1016/j.ejps.2021.105812. Epub 2021 Mar 20.
10. Kaur P, Jiang X, Duan J, Stier E. Applications of In Vitro-In Vivo Correlations in Generic Drug Development: Case Studies. AAPS J. 2015 Jul;17(4):1035-9. doi: 10.1208/s12248-015-9765-1.
11. Lutfor M, Al M, Nadarkhani F, Nezab M. Advanced Dissolution Testing for Novel Drug Formulations: Challenges, Emerging Methods, and Regulatory Perspectives. International Biomedi c a l Re s e a r ch, 2025;9(1):1-16. https://doi.org/10.25163/biomedical.9110245
12. Ajeh IJ, Hayatu GI, Ezekiel EI. Computational prediction of pharmacokinetic parameters as an in vitro approach for assessing paracetamol tablets for IVIVC; a strategy in COVID-19 disruptive ti m e s. Journal of Phytomedicine & Therapeutics, 2022; 21 (2): 835-845. https://dx.doi.org/10.4314/jopat.v21i2.6
13. Taha NF, Emara LH. Convolution- and Deconvolution-Based Approaches for Prediction of Pharmacokinetic Parameters of Diltiazem Extended-Release Products in Flow-Through Cell Dissolution Tester. AAPS PharmSciTech. 2022 Jul 26;23(6):202. doi: 10.1208/s12249-022-02361-2.
14. Rastogi V, Yadav P, Lal N, Rastogi P, Singh BK, Verma N, Verma A. Mathematical prediction of pharmacokinetic parameters-an in-vitro approach for investigating pharmaceutical products for IVIVC. Future Journal of Pharmaceutical Sciences, 2018; 4 (2): 175 - 184. https://doi.org/10.1016/j.fjps.2018.03.001
15. Bate R, Mathur A, Lever HM, Thakur D, Graedon J, Cooperman T, Mason P, Fox ER. Generics Substitution, Bioequivalence Standards, and International Oversight: Complex Issues Facing the FDA. Trends Pharmacol Sci. 2016 M a r ; 3 7 ( 3 ) : 1 8 4 - 1 9 1 . d o i : 10.1016/j.tips.2015.11.005
16. Vandy A, Conteh E, Lahai M, Kolipha-Kamara M, Marah M, Marah F, Suma KM, Mattia SC, Tucker KDS, Wray VSE, Koroma A, Lebbie AU. Physicochemical quality assessment of various brands of paracetamol tablets sold in Freetown Municipality. Heliyon. 2024 Feb 2;10(3):e25502. doi: 10.1016/j.heliyon.2024.e25502.
17. Cartwright AC (2016) The British pharmacopoeia, 1864 to 2014: medicines, international standards and the state. Routledge.
https://doi.org/10.4324/9781315614182
18. AlSwayeh R, Alvi SN, Hammami MM. Quality assessment of nine paracetamol 500 mg tablet brands marketed in Saudi Arabia. BMC Res Notes. 2021 Jun 30; 14(1): 254. doi: 10.1186/s13104-021-05672-y.
19. Malinowski H, Marroum P, Uppoor VR, Gillespie W, Ahn HY, Lockwood P, Williams R. FDA guidance for industry 1 extended release solid oral dosage forms: Development, evaluation, and application of in vitro/in vivo correlations. Dissolution Technology, 1997;4(4): 23–32. https://doi.org/10.14227/dt040497p23.
20. Bhosale AV, Hardikar SR, Patil N, Jagtap R, Jamdade N, Patel B. IVIVC and BCS: A regulatory perspective. Research Journal of Pharmacy & Technology, 2009;2(1):72-79.
21. Somani AA, Thelen K, Zheng S, Trame MN, Coboeken K, Meyer M, Schnizler K, Ince I, Willmann S, Schmidt S. Evaluation of changes in oral drug absorption in preterm and term neonates for Biopharmaceutics Classification System (BCS) class I and II compounds. Br J Clin Pharmacol. 2016 Jan;81(1):137-47. doi: 10.1111/bcp.12752.
22. Sjögren E, Thörn H, Tannergren C. In Silico Modeling of Gastrointestinal Drug Absorption: Predictive Performance of Three Physiologically Based Absorption Models. Mol Pharm. 2016 Jun 6 ; 1 3 ( 6 ) : 1 7 6 3 - 7 8 . d o i :
10.1021/acs.molpharmaceut.5b00861.
23. Vrbanac H, Trontelj J, Berglez S, Petek B, Opara J, Jereb R, Krajcar D, Legen I. The biorelevant simulation of gastric emptying and its impact on model drug dissolution and absorption kinetics. Eur J Pharm Biopharm. 2020 Apr;149:113-120. doi: 10.1016/j.ejpb.2020.02.002.
24. Muselík J, Komersová A, Kubová K, Matzick K, Skalická B. ACritical Overview of FDAand EMA Statistical Methods to Compare In Vitro Drug
Dissolution Profiles of Pharmaceutical Products. Pharmaceutics. 2021 Oct 15;13(10):1703. doi: 10.3390/pharmaceutics13101703.
25. Ruiz-Picazo A, Lozoya-Agullo I, GonzálezÁlvarez I, Bermejo M, González-Álvarez M. Effect of excipients on oral absorption process according to the different gastrointestinal segments. Expert Opin Drug Deliv. 2021 A u g ; 1 8 ( 8 ) : 1 0 0 5 - 1 0 2 4 . d o i : 10.1080/17425247.2020.1813108.
26. D'Arcy DM, Wacker MG, Klein S, Shah V, Burke MD, Hunter G, Xu H. In-vitro product performance of parenteral drug products: view of
the USP Expert Panel. Dissolution Technology, 2022;29(4):204-218.
27. Bermejo M, Hens B, Dickens J, Mudie D, Paixão P, Tsume Y, Shedden K, Amidon GL. A Mechanistic Physiologically -Based
Biopharmaceutics Modeling (PBBM) Approach to Assess the In Vivo Performance of an Orally Administered Drug Product: From IVIVC to IVIVP. Pharmaceutics. 2020 Jan 17;12(1):74. doi: 10.3390/pharmaceutics12010074.
28. Awemu GA, Anowi F, Ramos GF, Tejano GI. In vitro evaluation of quality control parameters of paracetamol tablets in Nigeria. World Journal of Pharmaceutical Science,2015; 4 (8):37-45 Available at : https://www.scribd.com/document/463941303/article-wjpps-1438352142para-important
29. Hamilton WL, Doyle C, Halliwell-Ewen M, Lambert G. Public health interventions to protect against falsified medicines: a systematic review of international, national and local policies. Health Policy Plan. 2016 Dec;31(10):1448-1466. doi: 10.1093/heapol/czw062.
30. National Agency for Food and Drug Administration and Control (2024) Misinformation on Nearly all Paracetamol Tablets in
Nigeria are Possibly Under Dosed. Available at: https://nafdac.gov.ng/nafdac-update-on-nearlyall-paracetamol-tablets-in-nigeria-are-possiblyunder-dosed/(Accessed: July 21, 2025).
31. Xie F, Ji S, Cheng Z. In vitro dissolution similarity factor (f2) and in vivo bioequivalence criteria, how and when do they match? Using a BCS class II drug as a simulation example. Eur J Pharm Sci. 2015 Jan 23; 66: 163 - 72. doi: 10.1016/j.ejps.2014.10.002.
Downloads
Views | PDF Downloads:
393
/ 231
Published
How to Cite
Issue
Section
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.


