Natural products-characterized moringa oleifera leaves methanolic extract and anti-diabetic properties mechanisms of its fractions in streptozotocin-induced diabetic rats
Keywords:
AMP-Kinase, Diabetes, α-amylase, Moringa oleifera,, Mechanism of actionAbstract
Background: The study characterized natural products and investigated the mechanisms of antidiabetic properties in Moringa oleifera leaves methanolic extract in Streptozotocin-induced diabetic male rats.
Methods: the natural bioactive compounds in difffferent fractions were obtained by column chromatography on 60-120 silica gel mesh and assayed using gas chromatography-mass spectrometric (GC-MS) technique. Also, possible anti-diabetic properties mechanisms of the fractions of extracts investigated using standard methods.
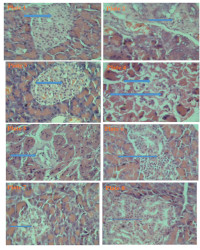
Results: Lethality study indicated safety of the extract at 5000mg/kgbwt. The GC- MS analysis of the methanolic extract revealed seven out of eleven common bioactive constituents with reported antidiabetic activity. Results of possible anti-diabetic properties mechanisms based on studied glucose homeostasis-related bio-indicators showed signifificant (P<0.05) increase in serum lipoprotein lipase, whole blood GLUT 1, liver homogenate G-6-P-DH, Intestine homogenate Na+ /K+ -ATPase, Muscle homogenate GLUT 4 and liver homogenate AMP-Kinase activities in rats administered with the drug (glibenclamide) and fractions 3,4,5 compared with the diabetic group. A signifificant (P<0.05) inhibition of serum α-amylase activity in rat administered drug and fractions 3,4,5 was observed when compared with diabetic control and fractions 1 and 2 . Histological outcomes of rats' pancreas corroborated these fifindings.
Conclusion: Thus, characterized natural bioactive constituents of M. oleifera fractions exerted concerted potent anti-diabetic activity in streptozotocin-induced diabetic rats by probable mechanisms involving concerted α-amylase inhibition, AMP-kinase and Lipo-protein lipase activation and glucose transport Proteins (GLUT-1 and GLUT-4) and liver homogenate Glucose-6-phosphate dehydrogenase up-regulation.
References
Gopinathan S, Naveenraj D. (2014) Antidiabetic activity of Clerodendrum phlomidis Linn. and Gymnema sylvestre Linn. in alloxan induced diabetic rats“ A comparative preclinical study. World Journal of pharmaceutical Research, 3(6): 1640-1675.
Wild S, Roqlic G, Green A, Sicree R and King H. (2004) Global prevalence of diabetes estimate for the year 2000 and projections for 2030. Diabetes Care, 27(5): 1047 - 1053. https://doi.org/10.2337/diacare.27.10.2568
World Health organization (WHO) (2018) Global report on diabetes. Geneva, Switzerland. World Health Organization.
https://www.who.int/publications/i/item/9789241565257
American Diabetes Association (2018) Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes. Diabetes Care, 41(1): 13 - 27, 73 - 85. https://doi.org/10.2337/dc18-S008
Periyar SS, Balu PM, Sathiya MPand Muragesan K. (2009) Antihyperglycemic effect of mangiterin in streptozotocin induced diabetic rats. Journal of Health Science, 55(2): 206-214.
Courtois P, Jijakli H, Ladriere L, Oguzhan B, Sener A, and Malaisse WJ. (2003) Pharmacodynamics, insulinotropic action and hypoglycemic effect of nateglinide and glibenclamide in normal and diabetic rats. International Journal of Molecular Medicine, 11, 105-109. https://doi.org/10.3892/ijmm.11.1.105
Ampa L, Watchara K and Tanaree J. (2013) Antihyperglycemic Properties of Moringa oleifera Lam. Aqueous Leaf Extract in Normal and Mildly Diabetic Mice, British Journal of Pharmacology and Toxicology, 4(3): 106 - 109.
https://doi.org/10.19026/bjpt.4.5371
Kasolo JN, Bimeya GS, Ojok L, Ochleng J, and Ogwal-Okeng JW. (2010) Phytochemical and uses of Moringa oleifera leave in Ugandan rural communities. Journal of Medicinal Plant Research, 4: 753 - 757. https://doi.org/10.3923/pjn.2016.397.405
Stahl, E. (1969) Thin Layer chromatography. A laboratory handbook. Springerverlag, Berlin, Herdberg, New York, Pp . 60 - 204. https://doi.org/10.1002/lipi.19710731210
National Research Council (1996) Institute for laboratory animal research; National Academies press (US), Washington DC. https://doi.org/10.17226/12910
Lorke D (1983) Anew approach to practical acute toxicity testing. Archives of Toxicology. 54:275-287. http://dx.doi.org/10.1007/BF01234480
Igwe OU, Okwu DE. (2013). GC–MS evaluation of bioactive compounds and antibacterial activity of the oil fraction from the seeds of Brachystegiaeury-coma (HARMS). Asian Journal Plant Science and Research. 3:47–54
Li WM, and McNeil JH. (1997) Quantitative methods for measuring the insulin-regulatable Glucose Transporter (GLUT). Journal of pharmacological and Toxicology methods: 38 (1): 1 - 1 0 . https://doi.org/10.1016/s1056-8719(97)00036-1
Gancedo JM and Gancedo C (1971) Fructose- 6-diphosphatase, Phosphofructokinase and glucose-6-phosphate dehydrogenase. Society Experimental Bio-Medical journal, 106: 607 - 609. https://doi.org/10.1007/BF00411787
Cheesbrough M (2005). District laboratory practice in tropical in tropical countries, 2nd edition, sheck wahTong printing Press Ltd. Hong Kong, 262-363. http://dx.doi.org/10.1017/CBO9780511543470
Korn, ED (1959) The assay of lipoprotein lipase in vivo and in vitro. Methods in Biochemistry Analiytical, 7: 145-192.
Suhail M and Rizvi SI. (1987) Red cell membrane Na++K+-ATPase in diabetes mellitus. Biochemistry and Biophysics Research Community, 146:179–186. https://doi.org/10.1016/0006-291x(87)90708-x
Palipoch S, Punsawad C (2013) Biochemical and histological study of rat liver and kidney injury induced by Cisplatin. Journal of toxicologic pathology. 26(3): 293 - 9. https://doi.org/10.1293/tox.26.293
Bamishaiye EI, Olayemi, FF, Awagu EF and Bamshaiye OM (2011) Proximate and Phytochemical Composition of Moringa oleifera Leaves at Three Stages of Maturation. Advanced Journal of Food Science and Technology, 3(4):
-237.
Kumar A, Lingadurai S, Jain A, Barman NR, (2010) Erythrina variegata Linn: A review on morphology, Phytochemistry and pharmacological aspects. Pharmacognosy Review, 4 (8): 147-152. https://doi.org/10.4103/0973-7847.70908
Berr aaouan A, Abid S, Bnouham M, Berraaouan A (2013) Antidiabetic oils. Current Diabetes. Rev.; 9:499–505.
Porter-Turner
MM, Skidmore JC, Khokher MA, Singh BM and Rea CA (2011). Relationship between erythrocyte GLUT1 function and membrane glycation in type 2 diabetes. British Journal of biomedical Science; 68 (4): 1-5.
Hsu F, I-min L, Daih-Huang K, Wang-Chuan C, Hu i-Chen S and Juei-Tang C (2003). Antihyperglycemic effect of puerain in streptozotocin-induced diabetic rats. Journal of Natural products, 66: 788-792.
Zhou G, Myers R, Li Y, Chen Y, Shen X, FenykMelody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ and Moller D E (2001) Role of AMP activated protein kinase in mechanism of metformin action. Journal of
Clinical Investment, 108 (8):1167−1174. https://doi.org/10.1172/JCI135050
Yueh-Hsiung K, Cheng-Hsiu L. and Chun-Ching S (2015). Anti-diabetic and Anti-hyperlipidemic Properties of a Triterpenoid Compound, Dehydroeburi coi c Ac id, from Antrodia camphorata in Vitro and in Streptozotocin Induced Mice. Journal of Agricultural and Food Chemistry, 63(46): 10140 - 10157. https://doi.org/10.1021/acs.jafc.5b04400
Kavitha R. (2014) Evaluation of Hypoglycemic effect of ethanolic extracts of leaf and fruit of T. dioica and leaf of C. ternatea in streptozotocin induced diabetic rats. International Journal of Pharmacology and Biological Sciences, 5(3):
-1068.
Ranganathan G, Li C and Kern PA (2000). The translational regulation of lipoprotein lipase in diabetic rats involves the 3-untranslated region of the lipoprotein lipase mRNA. The Journal of Biological Chemistry, 275(52): 40986-40991.
https://doi.org/10.1074/jbc.M008775200
Najafian M (2015) The Effects of Curcumin on Alpha Amylase in Diabetics Rats, Zahedan Journal of Research in Medical Sciences;17(12):e5198. https://doi.org/10.17795/zjrms-5198
Grindley PB, Omoruyi FO, Asemota HN, Morrison EY (2002). Carbohydrate digestion and intestinal ATPases in streptozotocin-induced diabetic rats fed extract of yam (Dioscorea cayenensis) or dasheen (Colocasiaesculenta).
Nutrition Research, 22(3), 333-341
Views | PDF Downloads:
961
/ 455
/ 0
Published
How to Cite
Issue
Section
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.


