Ameliorative influence of vildagliptin on genetic model of neurodegenerative diseases in Drosophila melanogaster
Keywords:
Drosophila melanogaster, vildagliptin, UAS-GAL4 system, Alzheimer's disease, Parkinson disease, negative geotaxisAbstract
Background: Neurodegenerative disorders (ND) are characterized by progressive loss of selectively vulnerable populations of neurons, which contrasts with select static neuronal loss. Self-association of amyloid-beta (Aβ) or α-synuclein peptides into fibrils and/or plaque like aggregates causes neurotoxicity. Hence, identification of specific compounds that either inhibit the formation of Aβ or α-syn-fibrils makes an appealing therapeutic strategy in the development of drugs. In the present study, we investigated the protective effect of vildagliptin (VDG) (oral hypoglycemic agent) on genetic models of ND in Drosophila melanogaster.
Methods: The disease causing human Aβ42 peptide or α-syn was expressed pan-neuronally (elav-GAL4) or dopamine neurons (DDC-GAL4) using the UAS-GAL4 system. Flies were either grown in food media with or without vildagliptin (1, 5, or 10µM). This was followed by fecundity, larva motility and negative geotaxis assay (climbing) as a measure of neurodegeneration.
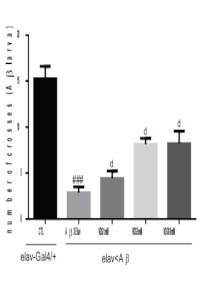
Results: Elav-Gal4<Aβ flies showed significant decrease in larva contraction and motility indicative of neurodegeneration. However, flies grown on vildaglitptin (VDG) showed dose-dependent and significant increase in larva motility in comparison with flies grown on food media only. Interestingly, the treatments did not affect fecundity when compared with normal food control. Moreover, flies grown on VDG showed no significant change in lifespan. DDC-GAL4<α-syn flies showed significant decrease in larva motility and climbing activity which was ameliorated by supplementation of flies food media with VDG suggestive of neuroprotective activity.
Conclusion: Findings from this study showed that vildagliptin is a potential neuroprotective agent in genetic or familial forms of neurodegeneration.
References
Bertram L, Tanzi RE. (2005) The genetic epidemiology of neurodegenerative disease. Journal of Clinical Investigation. 115(6): 1449 - 57. https://doi.org/10.1172/JCI24761
Coppedè F, Mancuso M, Siciliano G, Migliore L, Murri L . (2006) Genes and the environment in neurodegeneration. Biosci Rep. 26(5):341-67. https://doi.org/10.1007/s10540-006-9028-6
Ishola I.O, Badru A, Ofi E.O, Akinleye M.O, Adeyemi O.O (2021) Ameliorative influence of atorvastatin in transgenic Drosophila Melanogaster model of neurodegenerative diseases Nigerian Journal of Pharmacy 55 (1) 40-45. https://doi.org/10.51412/psnnjp.2021.7
Ermilov VV, Nesterova AA. (2016) β-amyloidopathy in the Pathogenesis of Age-Related Macular Degeneration in Correlation with Neurodegenerative Diseases. Advanced Experimental Medicine Biology 854:119-25. doi: 10.1007/978-3-319-17121-0_17.
Busson D, Pret AM. (2007) GAL4/UAS targeted gene expression for studying Drosophila Hedgehog signaling. Methods in Molecular Biology. 397:161-201. https://doi.org/10.1007/978-1-59745-516-9_13
Abdelsalam RM, Safar MM. (2015) Neuroprotective effects of vildagliptin in rat rotenone Parkinson's disease model: role of RAGE-NFκB and Nrf2-antioxidant signaling pathways. Journal of Neurochemistry. 133(5):700-7. https://doi.org/10.1111/jnc.13087
El Batsh MM, El Batch MM, Shafik NM, Younos IH (2015) Favorable effects of vildagliptin on metabolic and cognitive dysfunctions in streptozotocin-induced diabetic rats. European Journal of Pharmacology 769:297-305. https://doi.org/10.1016/j.ejphar.2015.11.033
El-Marasy SA, Abdel-Rahman RF, Abd-Elsalam RM. (2018) Neuroprotective effect of vildagliptin against cerebral schemia in rats. Naunyn Schmiedebergs Archival Pharmacology 391 (10): 1133 - 1145. https://doi.org/10.1007/s00210-018-1537-x
Ma QH, Jiang LF, Mao JL, Xu WX, Huang M. (2018) Vildagliptin prevents cognitive deficits and neuronal apoptosis in a rat model of Alzheimer's disease. Molecular Medical Report. 17(3): 4113 - 4119. https://doi.org/10.3892/mmr.2017.8289
Jain S, Sharma B. (2015) Neuroprotective effect of selective DPP-4 inhibitor in experimental vascular dementia. Physiology and Behavior. 152(Pt A):182-93. https://doi.org/10.1016/j.physbeh.2015.09.007
M'Angale PG, Staveley BE. (2016) Bcl-2 homologue Debcl enhances α-synuclein-induced phenotypes in Drosophila. Peer J. 15; 4: e2461. https://doi.org/10.7717/peerj.2461
Gadad BS, Britton GB, Rao KS. (2011) Targeting oligomers in neurodegenerative disorders: lessons from α-synuclein, tau, and amyloid-β peptide. Journal of Alzheimer Disease. 24 Suppl 2: 223 - 32. https://doi.org/10.3233/JAD-2011-110182
Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nature Review of Molecular Cell Biology, 2007 Feb;8(2):101-12. https://doi.org/10.1038/nrm2101
Shaltouki A, Hsieh CH, Kim MJ, Wang X. (2018) Alpha-synuclein delays mitophagy and targeting Miro rescues neuron loss in Parkinson's models. Acta Neuropathology 136(4): 607 - 620. https://doi.org/10.1007/s00401-018-1873-4
Vicente Miranda H, Szego ÉM, Oliveira LMA, Breda C, Darendelioglu E, de Oliveira RM, et al. (2017) Glycation potentiates α-synuclein-associated neurodegeneration in synucleinopathies. Brain. 140 (5):1399-1419. https://doi.org/10.1093/brain/awx056
Loveless J, Webb B. (2018) A Neuromechanical Model of Larval Chemotaxis. Integrative Computational Biology 58(5):906-914. https://doi.org/10.1093/icb/icy094
Chakraborty R, Vepuri V, Mhatre SD, Paddock BE, Miller S, Michelson SJ (2011) Characterization of a Drosophila Alzheimer's disease model: pharmacological rescue of cognitive defects. PLoS One. 6(6):e20799. https://doi.org/10.1371/journal.pone.0020799
Mudher A, Shepherd D, Newman TA, Mildren P, Jukes JP, Squire A, Mears A, Drummond JA, Berg S, MacKay D, Asuni AA, Bhat R, Lovestone S. (2004) GSK-3beta inhibition reverses axonal transport defects and behavioural phenotypes in Drosophila. Molecular Psychiatry. 9(5): 522 - 30. https://doi.org/10.1038/sj.mp.4001483
Folwell J, Cowan CM, Ubhi KK, Shiabh H, Newman TA, Shepherd D, Mudher A. A (2010) exacerbates the neuronal dysfunction caused by human tau expression in a Drosophila model of Alzheimer's disease. Experimental Neurology. 223 (2): 401 - 9. https://doi.org/10.1016/j.expneurol.2009.09.014
Ram KR, Wolfner MF. (2007) Sustained post-mating response in Drosophila melanogaster requires multiple seminal fluid proteins. PLoS Genetics. 3(12):e238. https://doi.org/10.1371/journal.pgen.0030238
Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science. 2010 Apr 16; 328 (5976): 321 - 6. https://doi.org/10.1126/science.1172539
Zhang S, Feng R, Li Y, Gan L, Zhou F, Meng S, Li Q, Wen T. (2018) Degradation of alpha-synuclein by dendritic cell factor 1 delays neurodegeneration and extends lifespan in Drosophila. Neurobiology of Aging. 67:67-74. https://doi.org/10.1016/j.neurobiolaging.2018.03.010
Views | PDF Downloads:
676
/ 220
/ 0
Published
How to Cite
Issue
Section
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.


