Vanillic Acid Ameliorates Diethyl Phthalate and Bisphenol S -Induced Cardiotoxicity in rats via abrogating oxidative Stress.
https://doi.org/10.51412/psnnjp.2023.21
Keywords:
Bisphenol S, diethyl phthalate, vanillic acid, cardiotoxicity, oxidative stressAbstract
Background: Neglected home producing toxicants (NHPT) are the endocrine disruptors affecting biological systems. We investigated the effect of vanillic acid on diethyl phthalate (DEP) and bisphenol S (BPS)-induced cardio-toxicity and oxidative stress using rat model.
Methods: Rats were exposed to DEP (50 mg/kg) and BPS (50 mg/kg) and treated with vanillic acid (25 and 50 mg/kg) by oral gavage for twenty-one days. Afterwards, they were sacrificed.
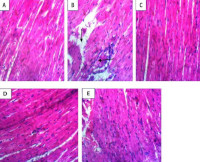
Results: Vanillic acid treatment significantly (p < 0.05) protected cardiac tissues architecture by abolishing the DEP+BPS-induced decrease in the activity of all the enzymatic antioxidants. Co-treatment with vanillic acid remarkably (p < 0.05) reversed DEP+BPS-induced decreases in glutathione levels, GSH, CAT, GPx, and SOD activities in cardiac tissues, while attenuating DEP+BPS mediated increase in cardio-oxidative damage markers (MDA,AOPP, and NO as well as increase activity of arginase and PDE-5.
Conclusion: Vanillic acid may have exert a cardio-protective effect against DEP+BPS induced cardiotoxicity by decreasing oxidative stress via its antioxidant, free radical scavenging effect.
References
Lirdprapamongkol K. Sakurai H, Kawasaki, N, Min-king C, Saitoh Y, Aozuka Y, et al. (2005) Vanillin suppresses in vitro invasion and in vivo metastasis of mouse breast cancer cells. European Journal of P h a r m a c e u t i c a l S c i e n c e s . 2 5 ( 1 ) : 5 7 - 6 5 . hƩps://doi.org/10.1016/j.ejps.2005.01.015
Chou TH, Ding H.Y, Hung W. J, Liang CH (2010) Antioxidative characteristics and inhibition of α‐melanocyte‐stimulating hormone‐stimulated melanogenesis of vanillin and VA from Origanum vulgare. Expermental Dermatology. 19(8):742-750. https://doi.org/10.1111/j.1600-0625.2010.01091.x
Lirdprapamongkol K, Kramb JP, Suthiphongchai T, Surarit R, Srisomsap C, Dannhardt G. (2009) Vanillic acid suppresses metastatic potential of human cancer cells through PI3K inhibition and decreases angiogenesis in vivo. Journal of Agriculture and Food C h e m i s t r y . 5 7 ( 8 ) : 3 0 5 5 - 3 0 6 3 . https://doi.org/10.1021/jf803366f
Amin FU, Shah SA, Kim MO. (2017) Vanillic acid attenuates Aβ 1-42-induced oxidative stress and cognitive impairment in mice. Sciences Repository. 18(1):1-15. https://doi.org/10.1038/srep40753
Dianat M, Hamzavi GR, Badavi M, Samarbafzadeh A. (2015) Effect of vanillic acid on ischemiareperfusion of isolated rat heart: Hemodynamic parameters and infarct size assays. Indian Journal of Experimental Biology. 53(1):641-646.
Kapanen, A., Stephen, J. R., Brüggemann, J., Kiviranta, A., White, D. C., and Itävaara, M. (2007) Diethyl phthalate in compost: ecotoxicological effects and response of the microbial community. C h e m o s p h e r e , 6 7 , 2 2 0 1 - 2 2 0 9 . https://doi.org/10.1016/j.chemosphere.
Weaver, J. A., Beverly, B. E., Keshava, N.,Mudipalli, A., Arzuaga, X., Cai, C., and Yost, E. E. (2020) Hazards of diethyl phthalate (DEP) exposure:A systematic review of animal toxicology studies. E n v i r o n m e n t I n t e r n a t i o n a l . 1 0 5 8 4 8 . hƩps://doi.org/10.1016/j.
Kang J. H, Katayama Y, and Kondo F. (2006) Biodegradation or metabolism of bisphenol A: From microorganisms to mammals. Toxicology. 217: 81 – 90.
Brede C, Fjeldal P, Skjevrak I. and Herikstad H. (2003) Increased migration levels of bisphenol A from polycarbonate baby bottles after dishwashing, boiling and brushing. Food Additives and Contaminants. 20: 684 – 689.
Huang I. J, Lian T. W, Wang L, LoY. H, and Wu M. J (2008) Fisetin, morin and myricetin attenuate CD36 expression and oxLDL uptake in U937-derived macrophages. Biochimicaet Biophysical Acta. 1781: 6 0 1 - 6 0 9 . https://doi.org/10.1016ij.bbalip.2008.06.009
Lee M. H, Cha H. J, Choi E. O, Han M. H, Kim S. O, Kim G. Y, and Choi Y. H. (2017) Antioxidant and cytoprotective effects of morin against hydrogen peroxide-induced oxidative stress are associated with the induction of Nrf-2-mediated HO-1 expression in V79-4 Chinese hamster lung fibroblasts. International journal of molecular medicine. 39:672-680. https://doi.org /10.1016/j.bbalip.2008.06.009
Yu S, Liu X, Yu D, Changyong E, and Yang J. (2020) Morin protects LPS-induced mastitis via inhibiting NLRP3 inflammasome and NF-κB signaling pathways. Inflammation. 43: 1293-1303. (ISSN: 1573-2576)
Verma V. K, Malik S, Narayanan S. P, Mutneja E, Sahu A. K, Bhatia J, and Arya D. S. (2019) Role of MAPK/NF-κB pathway in cardioprotective effect of Morin in isoproterenol induced myocardial injury in rats. Molecular biology reports. 46:1139-1148.
Bachewal P, Gundu C, Yerra V. G, Kalvala A. K, Areti A, and Kumar A. (2018) Morin exerts neuroprotection via attenuation of ROS induced oxidative damage and neuroinflammation in experimental diabetic neuropathy. Biofactors, 44:109- 122. https://doi.org /10.1002/biof.1397
Paoli P, Cirri P, Caselli A, Ranaldi F, Bruschi G, Santi A, and Camici G. (2013) The insulin-mimetic effect of Morin: A promising molecule in diabetes treatment. Biochimicaet Biophysica Acta (BBA)- General Subjects. 1830:3102-3111.16 https://doi.org/ 10.1016/j.bbagen.2013.01.017
E. Maestri, (2021) The 3Rs principle in animal experimentation: a legal review of the state of the art in Europe and the case in Italy, Biotechnology 10: 9–15, https://doi.org /10.3390/biotech10020009
Moron M. S, Depierre J. W, and Mannervik B. (1979) Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochimica et biophysica acta (BBA)-general s u b j e c t s , 5 8 2 ( 1 ) : 6 7 - 7 8 . hƩps://doi.org/10.1016/0304-4165(79)90289-7
Ellman G. L, (1959) Tissue sulfhydryl groups. Archives of biochemistry and biophysics, 82(1):70- 77. hƩps://doi.org/10.1016/0003-9861(59)90090- 6
Zou G. L, Gui X. F, Zhong X. L, and Zhu Y. F. (1986) Improvements in pyrogallol autoxidation method for the determination of SOD activity. Prog. Biochem.Biophys, 4: 71-73.
Hadwan M. H, and Abed H. N. (2016) Data supporting the spectrophotometric method for the estimation of catalase activity. Data in brief, 6: 194- 199. https://doi.org /10.1016/j.dib.2015.12.012
Rao M. N. A. (1997) Nitric oxide scavenging by c u r c u m i n o i d s . J o u r n a l o f p h a r m a c y a n d P h a r m a c o l o g y , 4 9 ( 1 ) : 1 0 5 - 1 0 7 . https://doi.org/10.1111/j.2042-7158.
Witko-Sarsat V, Friedlander M, CapeillèreBlandin C, Nguyen-Khoa T, Nguyen A. T, Zingraff J, and Descamps-Latscha B. (1996) Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney international, 49(5): 1304-1313. https://doi.org/ 10.1038/ki.1996.186
Octavia Y, Tocchetti C.G, Gabrielson K. L, Janssens S, Crijns H. J, Moens A. L. (2012) Doxorubicininduced cardiomyopathy: from molecular mechanisms to therapeutic strategies. Journal of Molecular Cell Cardiology. 52(6):1213- 1225. https://doi.org/ 10.1016/j.yjmcc.2012.03.006
Calixto-Campos C; Carvalho T.T; Hohmann M.SN; Pinho-Ribeiro F.A; Fattori V; Manchope M.F; Zarpelon A.C; Baracat M.M; Georgetti S.R; Casagrande R; and Verri W.A. (2015) Vanillic Acid Inhibits Inflammatory Pain by Inhibiting Neutrophil Recruitment, Oxidative Stress, Cytokine Production, and NFκB Activation in Mice. Journal of National . P r o d u c t i o n . 7 8 : 1 7 9 9 – 1 8 0 8 . https://doi.org/10.1021/acs.jnatprod.5b00246
Wu G, Fang Y. Z, Yang S, Lupton J. R, and Turner N. D. (2004) Glutathione metabolism and its implications for health. The Journal of nutrition, 1 3 4 ( 3 ) : 4 8 9 - 4 9 2 . h t t p s : / / d o i . o r g / 10.1093/jn/134.3.489
Muthukumar K, Rajakumar S, Sarkar M. N, and Nachiappan V. (2011) Glutathione peroxidase3 of Saccharomyces cerevisiae protects phospholipids during cadmium-induced oxidative stress. Antonie v a n L e e u w e n h o e k , 9 9 ( 4 ) : 7 6 1 - 7 7 1 . https://doi.org/10.1007/s10482-011-9550-9
Marrocco I, Altieri F, and Peluso I. (2017) Measurement and clinical significance of biomarkers of oxidative stress in humans. Oxidative medicine and c e l l u l a r l o n g e v i t y , https://doi.org/10.1155/2017/6501046
Chelikani P, Fita I, and Loewen P. C. (2004) Diversity of structures and properties among catalases. Cellular and Molecular Life Sciences CMLS, 61(2): 192-208. https://doi.org/10.1007/s00018-003-3206- 5
Förstermann U. (2010) Nitric oxide and oxidative stress in vascular disease. Pflügers Archiv-European Journal of Physiology, 459(6): 923-939.
Preiser J. C. (2012) Oxidative stress. Journal of Parenteral and Enteral Nutrition, 36(2):147-154. hƩps://doi.org/10.1177/0148607111434963
Davey M. W, Stals E, Panis B, Keulemans J, and Swennen R. L. (2005). High-throughput determination of malondialdehyde in plant tissues. Analytical biochemistry, 347(2): 201-207. https://doi.org/ 10.1016/j.ab.2005.09.041
Singh Z, Karthigesu I. P, Singh P, and Rupinder K. A. U R. (2014) Use of malondialdehyde as a biomarker for assessing oxidative stress in different disease pathologies: a review. Iranian Journal of Public Health, 43(3):7-16.
Ash D. E, Cox J. D, and Christianson D. W. ( 2 0 0 0 ) A rg i n a s e : a b i n u c l e a r m a n g a n e s e metalloenzyme. Metal ions in biological systems, 455- 476.
Ryoo S, Lemmon C. A, Soucy K. G, Gupta G, White A. R, Nyhan D, and Berkowitz D. E. (2006). Oxidized low-density lipoprotein–dependent endothelial arginase II activation contributes to impaired nitric oxide signaling. Circulation research, 9 9 ( 9 ) : 9 5 1 - 9 6 0 . https://doi.org/10.1161/01.RES.0000247034.24662.b 4
Kim J. H, Bugaj L. J, Oh Y. J, Bivalacqua T. J, Ryoo S, Soucy K. G, and Berkowitz D. E. (2009) Arginase inhibition restores NOS coupling and reverses endothelial dysfunction and vascular stiffness in old rats. Journal of applied physiology, 107(4):1249-1257
Pernow J, and Jung C. (2013) Arginase as a potential target in the treatment of cardiovascular disease: reversal of arginine steal?. Cardiovascular research, 98(3): 334-343.
Lu Z, Xu X, Hu X, Lee S, Traverse J. H, Zhu G, and Chen Y. (2010) Oxidative stress regulates left ventricular PDE5 expression in the failing heart. C i r c u l a t i o n , 1 2 1 ( 1 3 ) : 1 4 7 4 - 1 4 8 3 hƩps://doi.org/10.1161/CIRCULATIONAHA.109.9 06818
Marcela Škvařilová, Adam Bulava, David Stejskal, Sylya Adamovská, and Josef Bartek, (2005) Increased level of advanced oxidation products (AOPP) as a marker of oxidative stress in patients with acute coronary syndrome. Biomedical Papers, Med Fac University Palacky Olomouc Czech Republic. 149(10): 83-7
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 The Nigerian Journal of Pharmacy

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.

