Formulation and Evaluation of Fast Disintegrating Oral Tablets of Ibuprofen using Mannitol, PEG 4000, Cellactose and Sodium Starch Glycolate via Direct Compression
https://doi.org/10.51412/psnnjp.2022.32
Keywords:
orally disintegrating tablet, orodispersible tablet, fast dissolving, drug delivery, mouth disintegrating tabletAbstract
Background: The needs for improve drug bioavailability has led to the development of new excipients and combination of existing excipients to yield the desired purpose. This study seeks to develop an orally fast dissolving mouth disintegrating tablet of ibuprofen using PEG 4000, mannitol, cellactose and sodium starch glycolate as a preferred alternative for patient compliance and easy administration to geriatric patients.
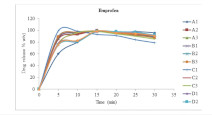
Method: Ten batches (A1, A2, A3, B1, B2, B3, C1, C2, C3, D1, D2, & D3,) of mixture of drug and various excipients combinations were developed and evaluated for powder properties. Tablets, equivalent of 500 mg of all the batches were compacted by direct compression method, on a Cadmach rotary tableting machine using 12.5 mm round-faced punches with pressure load of 10 KN. The compressed MDTs were evaluated for friability, weight variation, hardness, content uniformity, disintegration time, in vitro drug release and tablet oral taste.
Results: The results of powder flow properties and tablets analysis for all batchesA– D, batchA3, B1, B2, B3, and C1 showed promising characteristics for an ideal MDT with average powder flow rate, tablet disintegration time, T90%, crushing strength and taste as follow: A3 (1.58 gs-1, 2.09 s, 115 N, 7.5 min. and acceptable taste) respectively; B1 (1.54 gs-1, 2.01 s, 60 N, 6.5 min. and slight bitterness) respectively; B2 (1.58 gs-1, 1.52 s, 60 N, 5.5 min. and slight bitterness) respectively; B3 (1.61 gs-1, 1.05 s, 40 N, 5.0 min. and slight bitterness) respectively; C1 ( 1.72 gs-1, 0.57 s, 55 N, 4.0 min. and acceptable) respectively. Based on these characteristics, formulation batch C1 is more qualified for formulation of fast dissolving MDT.
Conclusion: The fast-disintegrating oral tablets of batch C1 possessed the ideal characteristics for physical stability and rapid release ofAPI which is an indication of improved bioavailability.
References
European Pharmacopoeia, 5th ed., Council of Europe, Strasbourg, 2006, p. 628.
Bandari, S. Mittapalli, R. K. and Gannu Rao, Y. M. (2008).Orodispersible tablet: An overview, Asian J.Pharm. 2 : 2–11. http://doi.org/10.4103/0973-8398.41557.
Agarwal, V. Kothari, B. H, Moe, D. V and Khankari, R. K. (2006). Drug delivery: Fast-dissolve Systems, in Encyclopedia of Pharmaceutical Technology (Ed. James Swarbrick), Informa Healthcare, New York 2006, pp. 1104–1114.
Sastry, S. V, Nyshadham, J. R and Fix, J.A(2000). Recent technological advances in oral drug delivery – a review, Pharm. Sci. Tech. Today 3 (2000) 138–145. http://doi.org/10.1016/S1461-5347(00)00247-9
Virely, P and Yarwood, R. (1990). Zydis – a novel fast dissolving dosage form, Manuf. Chem. 61 :36–37.
Wilson, C. G, Washington, N, Peach, J Murray, G. R and Kennerley, J (1987). The behavior of a fast dissolving dosage form (Expidet) followed by g-scintigraphy, Int. J.Pharm. 40: 119–123; http://doi.org/10.1016/0378-5173(87)90056-1.
Chandrasekhar, R, Hassan, Z., Alhusban, F., Smith, A. M and Mohammed, A (2009). R. The role of formulation excipients in the development of lyophilized fastdisintegrating tablets, Eur. J. Pharm. Biopharm. 72: 119–129; http://doi.org/10.1016/j.ejpb.2008.11.011.
Ahmed, I. S. Nafadi, M. M and Fatahalla, F. A (2006). Formulation of fast-dissolving ketoprofen tablet using freeze-drying in blisters technique, Drug Dev. Ind. Pharm. 32: 437–442; http://doi.org/10.1080/03639040500528913.
Kundu, S and Sahoo, P. K (2008). Recent trends in the developments of orally disintegrating tablet technology, Pharma Times 40: 11–15.
Proulx S.M, Melchiorre H.A(2001). New dosage forms lead to confusion. US Pharm.; 26: 68–70.
Pharmabiz K.V Pharmaceutical launches first product utilizing proprietary OraQuick delivery system; 2003 Jan.http://www.pharmabiz.com/article/detnews.asp?Arch=&articleid=13837 §ioned=14
Shah R.B, Tawakkul M.A, and Khan M.A. (2008). “Comparative Evaluation of Flow for Pharmaceutical Powders and Granules”, AAPS PharmSciTech,: 9 (1) 250- 258.
Lieberman H.A, Lachman L. (1989). Pharmaceutical dosage forms tablets. 2nd ed. New York: Marcel Dekker; 198: 9-15
United States pharmacopeia. National formulary USP/NF18. Peckville M.D., New York: The United States pharmacopeia convention (995)15: 133-145
Banker G.S, Anderson N.R. In: Lachman L, Lieberman H.A, Kanig J.L, eds. The Theory and Practice of Industrial Pharmacy. 3rd ed. Mumbai: Varghese Publishing House (1987) 293-399.
Arora P, Arora V. (2013). Orodispersible tablets: A comprehensive review. Int. J. Pharm. Sci. Res. 2(2):270-281
Views | PDF Downloads:
1424
/ 592
/ 0
Published
How to Cite
Issue
Section
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.


