Preclinical efficacy evaluation of two commercially available anti-snake venom against Naja nigricollis induced envenomation
Keywords:
EchiTab Plus-ICP, Naja nigricollis, Premium Antivenom, Snakebite, VenomAbstract
Background: The biochemical and immunological variations of snake venom components lead to many challenges in manufacturing appropriate anti snake venom (ASV). These variations have negatively impacted clinical outcomes due to the availability of ineffffective ASVs in countries where the manufacturing venom does not originate. There are reports of ineffffective ASVs exported to some African countries with public health and economic consequences on the already debilitating crisis. Recently, there have been calls and publications to draw the attention of policymakers and regional regulators on the need for preclinical and clinical data related to ASV, especially when manufactured from other regions. We therefore, screened the two most commercially available antisnake venom in Northern Nigeria against the most medically important cobra venom (N. nigricollis).
Methods: N. nigricollis venom was manually milked from fifive N. nigricollis captured from the wild and the LD50 of venom was determined using probit analysis. The effiffifficacy evaluation was conducted using the classical world health organization's preclinical mixing of venom/ASV methods on the lethal, hemorrhagic, hemolytic and necrotic effffect of the venom in mice and rabbit blood.
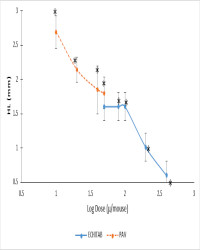
Result: The median lethal dose (LD50) of Naja nigricollis venom was estimated to be 1.0 mg/kg as calculated using Probit analysis. The two ASVs used for this study; EchiTab Plus-ICP and Premium Antivenom (PAV), provided protection (100%) against venom-induced lethality in mice except at the dose of 100l/mouse, where the PAV provided only 33% protection. All the administered doses of both EchiTab-Plus-ICP and PAV showed statistically signifificant reduction (p<0.001) in the mean hemorrhagic diameter when compared with the control group (19.12±1.95 mm). There was also signifificant reduction (p<0.001) in the mean necrotic diameter in all the groups compared to the control group (8.58±1.33 ml). Two dilutions of EchiTab-Plus-ICP (100 and 200 l) were able to signifificantly reduce (>50%) venom-induced hemolysis by 58 and 62% respectively, compared with the venom control group. On the other hand, such reduction was not observed with PAV.
Conclusion: The two most commercially available ASV in Northern Nigeria, EchiTab Plus-ICP and Premium Antivenom, were signifificantly (P > 0.01) effffective against lethality and venom-induced pathological parameters from Naja nigricollis envenoming, including; hemolysis, hemorrhagic and necrotic lesions, with EchiTab Plus-ICPshowing better activity.
References
World Health Organization. (2017) Annex 5. Guidelines for the production, control and regulation of snake antivenom immunoglobulins Replacement of Annex 2 of WHO Technical Report Series, No. 964. World Health Organization
Technical Report Series, (964), 197–388
Habib AG, & Brown NI. (2018) The snakebite problem and antivenom crisis from a health-economic perspective. Toxicon, 150; 115–123. https://doi.org/10.1016/j.toxicon.2018.05.009
Halilu S, Iliyasu G, Hamza M, Chippaux J.P., Kuznik A. and Habib A.G. (2019). Snakebite burden in Sub-Saharan Africa: estimates from 41 countries. Toxicon. 159: 1 – 4. https://doi.org/10.1016/j.toxicon.2018.12.002
Yusuf PO, Mamman M, Ajagun E, Suleiman MM, Kawu MU, Shittu M, Isa HI, Tauheed M., Yusuf A. (2015) Snakes Responsible for Bites in Norths-Eastern Nigeria – A Hospital-Based Survey. Journal of Environmental Science Ver. II, 9(9),
–2399
Habib AG, Gebi UI, Onyemelukwe GC. (2001) Snake bite in Nigeria. African Journal of Medicine and Medical Sciences. 30 (3): 171-8. PMID: 14510123.
Harrison RA, Oluoch GO, Ainsworth S, Alsolaiss J, Rowley P, Kalya S, Casewell NR. (2017) Preclinical antivenom-efficacy testing reveals potentially disturbing deficiencies of snakebite treatment capability in East Africa, PLOS Neglected Tropical Diseases| https://doi.org/10.1371/journal.pntd.0005969
Williams DJ, Gutiérrez JM, Calvete JJ, Wüster W, Ratanabanangkoon K, Paiva O, Brown HI, Casewell NR, Harrison RA, Rowley PD. (2011) Ending the drought: New strategies for improving the flow of affordable, effective antivenoms in Asia
and Africa. Journal of. Proteome, 74:1735–1767. https://doi.org/10.1016/j.jprot.2011.05.027
Stock RP, Massougbodji A, Alagón A, Chippaux JP. (2007) Bringing antivenoms to sub-Saharan Africa. Nature. Biotechnology, 25:173–177. https://doi.org/10.1038/nbt0207-173
Visser LE, Kyei-Faried S, Belcher DW, Geelhoed DW, van Leeuwen JS, van Roosmalen J. (2008) Failure of a new antivenom to treat Echis ocellatus snake bite in rural Ghana: The importance of quality surveillance. Transactions of the Royal Society of Tropical Medicine and Hygine. 102: 445 – 450. https://doi.org/10.1016/j.trstmh.2007.11.006
Bala AA, Jatau, AI, Yunusa I, Mohammed M, Mohammed AH, Isa AM, Wada AS, Gulma KA, Bello I, Malami S, Michael GC, & Chedi BA. (2021) Knowledge assessment of anti-snake venom among healthcare practitioners in northern Nigeria. Therapeutic advances in infectious disease, 8, 20499361211039379. https://doi.org/10.1177/20499361211039379
Markfarlane RG. (1967) Russell's Viper Venoms, 1953 – 1964. British. Journal of Haematology; 13, 437– 451
Theakston RD, Reid HA. (1983) Development of simple standard assay procedures for the characterization of snake venom. Bulletin of World Health Organization. 1983;61(6):949–56.
Gould LA, Lansley AB, Brown MB, Forbes B, Martin GP. (2000) Mitigation of surfactants erythrocyte toxicity by egy phosphatidylcholine. The Journal of Pharmacy Pharmacology, 52 (10): 1203 - 1209 https://doi.org/10.1211/0022357001777333
IBM Corp. Released (2020). IBM SPSS Statistics for Windows, Version 27.0. Armonk, NY: IBM Corp
Chippaux JP, Williams V, and White J. (1991) Snake venom variability: methods of study, results and interpretation. Toxicon: 29(11): 1279–1303. https://doi.org/10.1016/0041-0101(91)90116-9
Calmette A. (1896) The treatment of animals poisoned with snake venoms by the injection of anti-venomous serum. British Medical Journal, 1859, 399–400.
Gutiérrez JM, Solano G, Pla D, Herrera M, Segura Á, Vargas M, Villalta M, Sánchez A, Sanz L, Lomonte B, León G, & Calvete JJ. (2017) Preclinical Evaluation of the Efficacy of Antivenoms for Snakebite Envenoming: State-of-the-Art and Challenges Ahead. Toxins, 9(5), 163. https://doi.org/10.3390/toxins9050163
Adeyi AO, Ajisebiola SB, Esther OA, Chibuisi GA, Uchennaya GO. (2020) Antivenom activity of Moringa oleifera leave against pathophysiological alterations, somatic mutation and biological activities of Naja nigricollis venom, Scientific
African, (8) e00356, https://10.1016/j.sciaf. 2020.e00356
Oge O, Ike C, Agusi K, Chinwuba P, Ifebi HM, and Ezeokafor EN. (2018) Neutralization Potentials of Portulaca oleracea Leaf Extract against Naja nigricollis Venom Phospholipase A2. International Journal of Scientific & Engineering Research (9) 2 pp. 2229-5518
Abubakar MS, Sule MI, Pateh UU, Abdurahman EM, Haruna AK, Jahun BM. (2000) In vitro snake venom detoxifying action of the leaf extract of Guierasenegalensis, Journal of Ethnopharmacology (69)3pp 253 - 257
https://doi.org/10.1016/S0378-8741(99)00128-2
Abubakar MS, Balogun Abdurahman EM, Nok AJ, Shok M, Mohammed A, & Garba M. (2006) Ethnomedical Treatment of Poisonous Snakebites: Plant Extract Neutralized Naja nigricollis. Venom, Pharmaceutical Biology, 44:5, 343-348, https://doi.org/10.1080/13880200600746253
Isa HI, Ambali SF, Suleiman MM, Abubakar MS, Kawu MU, Shittu M, Yusuf PO, and Habibu B. (2015) Journal of Environmental Science, Toxicology and Food Technology (9)12 pp100-105
Williams HF, Hayter P, Ravishankar D, Baines A, Layfield HJ, Croucher L, & Vaiyapuri S. (2018) Impact of Naja nigricollis venom on the production of methaemoglobin. Toxins, 10(12), 539. https://doi.org/10.3390/toxins10120539
Segura A, Villalta M, Herrera M, León G, Harrison R, Durfa N, Nasidi A, Calvete JJ, Theakston RD, Warrell DA, Gutiérrez JM. (2010) Preclinical assessment of the efficacy of a new antivenom (EchiTAb-Plus-ICP) for the treatment of viper envenoming in sub-Saharan Africa. Toxicon; 55 (2 - 3): 369 - 74. https://doi.org/10.1016/j.toxicon.2009.08.010
Calvete JJ, Cid P, Sanz L, Segura A, Villalta M, Herrera M, León G, Harrison R, Durfa N, Nasidi A, Theakston RD, Warrell DA, & Gutiérrez JM. (2010) Antivenomi c a ss e ssment of the immunological reactivity of EchiTAb-Plus-ICP, an antivenom for the treatment of snakebite envenoming in sub-Saharan Africa. The American journal of tropical medicine and hygiene; 82(6), 1194 – 1201. https://doi.org/10.4269/ajtmh.2010.09-0733
Habib AG. (2013) Public health aspects of snakebite care in West Africa: perspectives from Nigeria. J Venom Anim Toxins Incl Trop Dis; 17: 19(1):27. https://doi.org/10.1186/1678-9199-19-27
Michael GC, Grema BA, Aliyu I, Mohammed AA, Teslim OL, Haliru I, Aminu GF, Fatima SG, Kennedy NK, Thomas DT, Abba KB, Emmanuel O. (2018) Knowledge of venomous snakes, snakebite first aid, treatment, and prevention among clinicians in northern Nigeria: a cross-sectional multicentre study. Transactions of Royal Society of Tropical Medicine and Hygiene; 112: 47–56 https://doi.org/10.1093/trstmh/try028
Bala AA, Jatau AI, Yunusa I, Mohammed M, Mohammed AH, Isa AM, Wada AS, Gulma KA, Bello I, Michael GC, Malami S, Chedi BZA. (2020) Knowledge assessment of snake antivenom among healthcare practitioners involving educational intervention in northern Nigeria: a study protocol. Therapeutic Advances in Drug Safety. 31; 11. https://doi.org/10.1177/2042098620935721
Views | PDF Downloads:
1515
/ 453
/ 0
Published
How to Cite
Issue
Section
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.


