Prevalence and Antibiogram of Healthcare-Associated Methicillin-Resistant <i>Staphylococcus aureus</i> (HA-MRSA) in a Public Tertiary Healthcare Facility (NH) in Enugu, Enugu State, Nigeria.
Keywords:
MRSA, HA-MRSA, antibiotic resistance, antibiotics, MARI, clinical samples, gentamycinAbstract
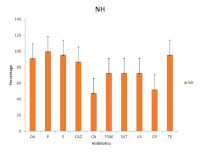
Methicillin Resistant Staphylococcus aureus (MRSA) remains a major cause of both community and healthcare-associated infections. This study was designed to determine the prevalence and antibiogram of healthcare-associated MRSA(HA-MRSA) in a public tertiary healthcare facility (NH) in Enugu, Enugu State, Nigeria. A total of 62 {(male-33; female- 29) clinical samples were obtained from NH in Enugu State. S. aureus was isolated, characterized and identified based on standard microbiological procedures. Antibiograms of isolated MRSA isolates were determined by the KirbyBauer disc diffusion technique according to the Clinical Laboratory Standards Institute (CLSI) guidelines. The prevalence of MRSA was higher amongst the isolates obtained from males (48.5%) than females (41.4%). The highest prevalence of MRSA in relation to age and sample source were obtained from {(31-45) and (46-60)} years and urine as (66.7%) and (42.6%), respectively. HAMRSA were highly resistant to penicillin (100%), tetracycline (95.6%), and erythromycin (95.6%), but moderately susceptible to gentamycin and ciprofloxacin. A mean multiple antibiotic resistance index (MARI) of 0.8 was observed in this study with 96% > 0.2. In conclusion, prevalence of HAMRSA was high in the study area and there is a need for more proactive measures to curb this public health menace before it escalates beyond control.
References
1. Jorge A, Schneider J, Unsleber S, Xia G, Mayer C, Peschel A. Staphylococcus aureuscounters phosphate limitation by scavenging wall teichoic acids from other staphylococci via the teichoicase GlpQ. J Biol Chem. 2018; 293(38): 1-16. doi: 10.1074/jbc.RA118.004584
2. Kruger W, Vielreicher S, Kapitan M, Jacobsen ID, Niemiec MJ. Fungal-Bacterial Interactions in Health and Disease. Pathogens. 8(2): 1-41. doi: 10.3390/pathogens8020070.
3. Lakhundi S, Zhang K. Methicillin-Resistant Staphylococcus aureus: Molecular Characterization, Evolution, and Epidemiology.
Clin Microbiol Rev, 2018 31(4):e00020-18. doi: 10.1128/CMR.00020-18
4. Bien J, Sokolova O, Bozko P. Characterization of Virulence Factors of Staphylococcus aureus: Novel Function of Known Virulence Factors That Are Implicated in Activation of Airway Epithelial Proinflammatory Response. J Pathol. 2011; 1 - 13. doi: 10.4061/2011/601905
5. Fisher EL, Otto M, Cheung G. Basis of Virulence in Enterotoxin-Mediated Staphylococcal Food Poisoning. Front Microbiol, 2018; 9: 436. doi: 10.3389/fmicb.2018.00436.
6. Myles IA, Datta SK. Staphylococcus aureus: an introduction. Semin Immunopathol, 2012; 34(2): 181-184. doi: 10.1007/s00281-011-0301-9.
7. Gnanamani A, Hariharan P, Paul- Satyaseela M. Staphylococcus aureus: Overview of Bacteriology, Clinical Diseases, Epidemiology, Antibiotic Resistance and Therapeutic Approach, Frontiers in Staphylococcus aureus, IntechOpen, 2017; doi: 10.5772/67338.
8. Garoy EY, Gebreab YB, Achila OO, Tekeste DG, Kesete R, Ghirmay R, Kiflay R, Tesfu T. Methicillin-Resistant Staphylococcus aureus (MRSA): Prevalence and Antimicrobial Sensitivity Pattern among Patients: A Multicenter Study in Asmara, Eritrea. Can J Infect Dis Med Mi c robiol, 2019; Arti c l e ID 8321834. doi:10.1155/2019/8321834.
9. World Medical Association (WMA). WMA declaration of Helsinki – Ethical Principles for Medical Research involving Human
th Participants.75 WMA General Assembly, Helsinki, Finland, October 2024.
10. Cheesbrough, M. District Laboratory Practice in nd Tropical Countries, Part two, 2 edn. Cambridge University Press, UK. 2010. Pp 143-180.
11. Ariom TO, Iroha IR, Moses IB, Iroha CS, Ude UI, Kalu A C. Detection and phenotypic characterization of methicillin-resistant
Staphylococcus aureus from clinical and community samples in Abakaliki, Ebonyi State, Nigeria. Afri Health Sci, 2019; 19(2): 2026-2035. doi: 10.4314/ahs.v19i2.26
12. Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Sus c eptibility Te sting; Twenty-Fourth Informational Supplement. CLSI Document M100-S24, Wayne, 2014; 34(1).
13. Moses IB, Esimone CO, Iroha IR, Ugbo EN, Nwuzo AC, Orji JO, Nwakaeze EA, Agumah NB, Emioye AA, Ukpai EG, Ogene LN. (2020). First Report on the Antibiotic Resistance Profiles and Virulence Genes of Staphylococcus pseudintermedius Colonizing Dogs and Humans in Abakaliki, South-East Nigeria. Res J Microbiol, 15: 22-34. doi: 10.3923/jm.2020.22.34
14. Ukpai EG, Chukwura EI, Moses IB, Ugbo EN, Agumah NB, Okata- Nwali OD, Anakwenze VN, Nnabugwu CC, Onadagu BO, Ogu GI, Igborgbor JC, Okoye CS. (2021). Prevalence and Antibiogram of Healthcare - Associated Methicillin-Resistant Staphylococcus aureus (HA-MRSA) in Ebonyi State, Nigeria. Int J Pharm Sci Rev and Res. 69(1):104 -111. doi:10.47583/ijpsrr.2021.v69i01.016
15. Onwubiko NE, Sadiq NM. Antibiotic sensitivity pattern of Staphylococcus aureusfrom clinical isolates in a tertiary health institution in Kano, Northwestern Nigeria. Pan African Med J, 2011; 8(1). doi: 10.4314/pamj.v8i1.71050
16. Abdullahi N, Iregbu K C. Methicillin-Resistant Staphylococcus aureus in a Central Nigeria Tertiary Hospital. Ann Trop Pathol, 2018; 9(1):6-10. https://doi.org/10.4103/atp.atp_37_17
17. Udeani TK, Onyebuchi CJ, Ikpenwa MC, Ezenwaka UR. Prevalence and Antibiotic Susceptibility Pattern of Methicillin Resistant Staphylococcus aureus in Burns and Pressure Ulcer Patients. Afr J Clin Exp Microbiol, 2016:17(2):130-139. doi:10.4314/ajcem.v17i2.9
18. Udobi CE, Obajuluwa, AF, Onaolapo, JA. “Prevalence and antibiotic resistance pattern of methicillin-resistant Staphylococcus aureus from an orthopaedic hospital in Nigeria,” BioMed Res Int, 2013: 4 pages, ID 860467. doi: 10.1155/2013/860467
19. Ugbo EN, Moses IB, Ugadu IO, Ugbo AI. (2021). Plasmid profiling and prevalence of methicillinresistant Staphylococcus aureus from patients in Abakaliki, Nigeria. Niger J Microbiol, 2021; 35(1): 5454-5463. https://www.researchgate.net/publication/353086471
20. Lunzen J, Altfeld M. Sex Differences in Infectious Diseases-Common but Neglected. J Infect Dis, 2014; 209(3)79-80. doi: 10.1093/infdis/jiu159.
21. Ike B, Ugwu MC, Ikegbunam MN, Nwobodo D, Ejikeugwu C, Thaddeus GT, Esimone, CO. (2016). Prevalence, Antibiogram and Molecular Characterization of Community-Acquired Methicillin-Resistant Staphylococcus aureus in AWKA, Anambra Nigeria. Open Microbiol J, 2016; 10: 211- 221. doi: 10.2174/1874285801610010211
22. Okon K, Uba ABP, Oyawoye OM, Yusuf IZ, Shittu AO, Blanc DLJ. Epidemiology and characteristic pattern of methicillin-resistant Staphylococcus aureus recovered from tertiary hospitals in Northeastern Nigeria. Int J Trop Med, 2011; 6(5):106-12.
https://www.researchgate.net/publication/286606626.
23. Shittu A, Oyedara O, Abegunrin F, Okon K, Raji A, Taiwo S, Ogunsola F, Onyedibe K, Elisha G. Characterization of methicillin-susceptible and resistant staphylococci in the clinical setting: a multicentre study in Nigeria. BMC Infect Dis, 2012; 12(1): 286. https://doi.org/10.1186/1471-2334-12-286
24. Terry AOA, Ogbolu DO, Mustapha JO, Akinbami R, Ajayi AO. The non-association of PantonValentine leukocidin and mecA genes in the genome of Staphylococcus aureus from hospitals in South Western Nigeria. Indian J Med Microbiol, 2012; 30: 159-64. https://doi.org/10.4103/0255-0857.96675
25. Ogbolu D, Alli AO, Bello LA, Ibrahim LA. (2015). Emergence of Vancomycin Intermediate Staphylococcus aureus (VISA) in clinical Isolates of from south Western Region of Nigeria. Int J Trop Dis Health, 2015; 10(4): 1-5. doi: 10.9734/IJTDH/2015/20642
26. Aminu AI, Abdullahi S, Usman MI. Detection of Methicillin Resistant Staphylococcus aureus (MRSA) from Hospital Instruments. UMYU J Microbiol Res, 2017; 2(1): 10 - 21. https://doi.org/10.47430/ujmr.1721.003
27. Wang W, Baloch Z, Jiang T, Zhang C, Peng Z, Li F, Fanning S, Ma A, XuJ. (2017). Enterotoxigenicity and Antimicrobial Resistance
of Staphylococcus aureus Isolated from Retail Food in China. Front in Microbiol 8:2256. https://doi.org/10.3389/fmicb.2017.02256.
28. Dilnessa T, Bitew A. Prevalence and antimicrobial susceptibility pattern of methicillin resistant Staphylococcus aureus isolated from clinical samples at Yekatit 12 Hospital Medical College, Addis Ababa, Ethiopia,” BMC Infect Dis, 2016; 16 (1): 398. https://doi.org/10.1186/s12879-016-1742-5
29. Nsofor CA, Nwokenkwo VN, Ohale CU. Prevalence and antibiotic susceptibility pattern of Staphylococcus aureus isolated from various clinical specimens in South East Nigeria. MedCrave Online J Cell Sci Rep, 2016; 3(2):1-5.
https://www.researchgate.net/publication/304355652
30. Ugwu MC, Mokwe N, Ejikeugwu PC, Gugu TH, Enemor EC, Eze CO, Ugwu BC. (2015). Antibiogram of Staphylococcus aureus from healthy school pupils in Agulu, Southeastern Nigeria. Int J Res Pharm Biosci, 2015; 2(4):5-9. doi: 10.2174/1874285801610010211
31. Adefurin A, Sammons H, Jacqz-Aigrain E, Choonara I. Ciprofloxacin Safety in Paediatrics: A systematic Review. Arch Dis Child, 2011; 2(9): 874 - 880. https://doi.org/10.1136/adc.2010.208843.
Downloads
Views | PDF Downloads:
841
/ 177
Published
How to Cite
Issue
Section
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.


