Anatomical and Functional Distribution of Brain Metalloproteins: Roles of Iron, Copper, Zinc, and Selenium and Their Implications in Some Neurological Disorders
Keywords:
Metalloproteins, Anatomical Distribution, Trace Metals, Neurodegeneration, Brain Metal ImbalanceAbstract
Background: Metalloproteins play crucial roles in brain physiology by mediating redox balance, neurotransmission, and cellular metabolism through interactions with trace metals. Understanding their regional distribution and functional relevance enhances insight into neurodegenerative disease mechanisms.
Objective: This systematic and comparative review examines the anatomical distribution, physiological functions, and neuropathological significance of iron-, copper-, zinc-, and seleniumdependent metalloproteins in the human brain.
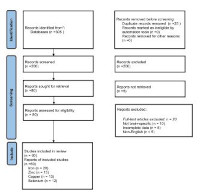
Methods: Following the PRISMA framework, literature published between 2010 and 2025 was systematically reviewed. Sixty studies met the inclusion criteria and were analysed for patterns of metalloprotein localisation and function across major brain regions.
Results: Iron-related proteins (ferritin, transferrin, DMT1) are predominant in the substantia nigra and basal ganglia, supporting oxygen transport and dopamine metabolism. Copper-binding enzymes (ceruloplasmin, SOD1, cytochrome c oxidase) are enriched in the cerebellum and hippocampus, promoting mitochondrial function. Zinc-associated proteins (ZnT3, metallothionein-III, MMP-9) dominate in the hippocampus and cortex, facilitating synaptic plasticity, while selenium-based selenoproteins (GPX4, SELENOP, TrxR) are concentrated in the cerebellum and hypothalamus, regulating oxidative defence.
Conclusion: Overlapping expression zones such as the substantia nigra (iron and copper) and hippocampus (zinc and selenium) indicate shared redox and signalling roles. Metalloproteins are essential for maintaining neuroanatomical integrity, and their dysregulation contributes to regionspecific neurodegenerative disorders, highlighting their potential as diagnostic and therapeutic biomarkers.
References
1. Ibrahim IH. Metalloproteins and metalloproteomics in health and disease. In: Advances in Protein Chemistry and Structural Biology [Internet]. Elsevier; 2024 [cited 2025 Jul 1]. p. 123–76.
2. Prohaska JR. Functions of trace elements in brain metabolism. Physiol Rev. 1987 Jul;67(3):858–901.
3. Rouault TA, Cooperman S. Brain Iron Metabolism. Semin Pediatr Neurol. 2006 Sep;13(3):142–8.
4. Hare D, Ayton S, Bush A, Lei P. A delicate balance: Iron metabolism and diseases of the brain. Front Aging Neurosci [Internet]. 2013 [cited 2025 Jun 10];5.
5. Frederickson CJ, Koh JY, Bush AI. The neurobiology of zinc in health and disease. Nat Rev Neurosci. 2005 Jun;6(6):449–62.
6. Ward RJ, Zucca FA, Duyn JH, Crichton RR, Zecca L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. 2014 Oct;13(10):1045–60.
7. Matak P, Matak A, Moustafa S, Aryal DK, Benner EJ, Wetsel W, et al. Disrupted iron homeostasis causes dopaminergic neurodegeneration in mice. Proc Natl Acad Sci. 2016 Mar 29;113(13):3428–35.
8. Labunskyy VM, Gladyshev VN. Role of Reactive Oxygen Species-Mediated Signaling in Aging. Antioxid Redox Signal. 2013 Oct 20;19(12):1362–72.
9. Gh Popescu BF, Nichol H. Mapping Brain Metals to Evaluate Therapies for Neurodegenerative Disease: Mapping Brain Metals to Evaluate Therapies for Neurodegenerative Disease. CNS Neurosci Ther. 2011 Aug;17(4):256–68.
10. Dusek P, Litwin T, Czlonkowska A. Wilson Disease and Other Neurodegenerations with Metal A c c u m u l a t i o n s . N e u r o l C l i n . 2 0 1 5 Feb;33(1):175–204.
11. Takeda A. Brain Function and Pathophysiology 2+ Focused on Zn Dynamics. YAKUGAKU ZASSHI. 2022 Aug 1;142(8):855–66.
12. Solovyev N, Drobyshev E, Blume B, Michalke B. Selenium at the Neural Barriers: A Review. Front Neurosci. 2021 Feb 5;15:630016.
13. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J Clin Epidemiol. 2021 Jun;134:178–89.
14. Reddy VS, Bukke S, Dutt N, Rana P, Pandey AK. A systematic review and meta-analysis of the circulatory, erythrocellular and CSF selenium levels in Alzheimer's disease: A metal meta-analysis (AMMA study-I). J Trace Elem Med Biol. 2017 Jul;42:68–75.
15. Verma S, Goel T, Tanveer M. Quantitative Susceptibility Mapping in Cognitive Decline: A Review of Technical Aspects and Applications [Internet]. arXiv; 2022 [cited 2025 Jul 3].
16. Sian‐Hülsmann J, Mandel S, Youdim MBH, Riederer P. The relevance of iron in the pathogenesis of Parkinson's disease. J Neurochem. 2 0 11 Sep;118(6):939–57.
17. Lee DW, Andersen JK. Iron elevations in the aging Parkinsonian brain: a consequence of impaired iron homeostasis? J Neurochem. 2010 Jan;112(2):332–9.
18. Liu Z, Shen H cong, Lian T hong, Mao L, Tang S xian, Sun L, et al. Iron deposition in substantia nigra: abnormal iron metabolism, neuroinflammatory mechanism and clinical relevance. Sci Rep. 2017 Nov 2;7(1):14973.
19. Dexter DT, Jenner P. Parkinson disease: from pathology to molecular disease mechanisms. Free Radic Biol Med. 2013 Sep;62:132–44.
20. Schipper DA, Schipper HM. Neurodegeneration with Brain Iron Accumulation. In: Pantopoulos K, editor. Iron Metabolism in Human Health and Disease [Internet]. Cham: Springer Nature Switzerland; 2025 [cited 2025 Jul 3]. p. 291–309. (Advances in Experimental Medicine and Biology; vol. 1480).
21. Shayeghi M, Latunde-Dada GO, Oakhill JS, Laftah AH, Takeuchi K, Halliday N, et al. Identification of an Intestinal Heme Transporter. Cell. 2005 Sep;122(5):789–801.
22. Luck AN, Mason AB. Transferrin-Mediated Cellular Iron Delivery. In: Current Topics in Membranes [Internet]. Elsevier; 2012 [cited 2025 Jul 5]. p. 3–35.
23.Luck AN, Mason AB. Structure and dynamics of drug carriers and their interaction with cellular receptors: Focus on serum transferrin. Adv Drug Deliv Rev. 2013 Jul;65(8):1012–9.
24. Tortorella S, Karagiannis TC. Transferrin ReceptorMediated Endocytosis: A Useful Target for Cancer Therapy. J Membr Biol. 2014 Apr;247(4):291–307.
25. Raha AA, Biswas A, Henderson J, Chakraborty S, Holland A, Friedland RP, et al. Interplay of Ferritin Accumulation and Ferroportin Loss in Ageing Brain: Implication for Protein Aggregation in Down Syndrome Dementia, Alzheimer's, and Parkinson's Diseases. Int J Mol Sci. 2022 Jan 19;23(3):1060.
26. Raha AA, Vaishnav RA, Friedland RP, Bomford A, Raha-Chowdhury R. The systemic iron-regulatory proteins hepcidin and ferroportin are reduced in the brain in Alzheimer's disease. Acta Neuropathol Commun. 2013 Dec;1(1):55.
27. Ingrassia R, Garavaglia B, Memo M. DMT1 Expression and Iron Levels at the Crossroads Between Aging and Neurodegeneration. Front Neurosci. 2019 Jun 5;13:575.
28. Ingrassia R, Memo M, Garavaglia B. Ferrous Iron Upregulation in Fibroblasts of Patients with Beta Propeller Protein-Associated Neurodegeneration (BPAN). Front Genet [Internet]. 2017 Feb 17 [cited 2025 Jul 6];8.
29. An Y, Li S, Huang X, Chen X, Shan H, Zhang M. The Role of Copper Homeostasis in Brain Disease. Int J Mol Sci. 2022 Nov 10;23(22):13850.
30. Faller P, Hureau C. A Bioinorganic View of Alzheimer's Disease: When Misplaced Metal Ions (Re)direct the Electrons to the Wrong Target. Chem – Eur J. 2012 Dec 7;18(50):15910–20.
31. Gromadzka G, Tarnacka B, Flaga A, Adamczyk A. Copper Dyshomeostasis in Neurodegenerative Diseases—Therapeutic Implications. Int J Mol Sci. 2020 Dec 4;21(23):9259.
32. Bhattacharjee A, Ghosh S, Chatterji A, Chakraborty K. Neuron-glia: understanding cellular copper homeostasis, its cross-talk and their contribution towards neurodegenerative diseases. Metallomics. 2020 Dec 1;12(12):1897–911.
33. Rihel J. Copper on the brain. Nat Chem Biol. 2018 Jul;14(7):638–9.
34. Davies KM, Hare DJ, Cottam V, Chen N, Hilgers L, Halliday G, et al. Localization of copper and copper transporters in the human brain. Metallomics. 2013;5(1):43–51.
35. Wang H, Wang M, Wang B, Li M, Chen H, Yu X, et al. The distribution profile and oxidation states of biometals in APP transgenic mouse brain: dyshomeostasis with age and as a function of the development of Alzheimer's disease. Metallomics. 2012;4(3):289.
36. Montes S, Rivera-Mancia S, Diaz-Ruiz A, TristanLopez L, Rios C. Copper and Copper Proteins in Parkinson's Disease. Oxid Med Cell Longev. 2014;2014:1–15.
37. Cheli VT, Sekhar M, Santiago González DA, Angeliu CG, Denaroso GE, Smith Z, et al. The expression of ceruloplasmin in astrocytes is essential for postnatal myelination and myelin maintenance in the adult brain. Glia. 2023 Oct;71(10):2323–42.
38. Rowlands BD, Trist BG, Karozis C, Schaffer G, Mor D, Harwood R, et al. Copper supplementation mitigates Parkinson-such as wild-type SOD1 pathology and nigrostriatal degeneration in a novel mouse model. Acta Neuropathol Commun. 2025 Jun 25;13(1):133.
39. Opačić M, Ristić AJ, Sokić D, Baščarević V, Raičević S, Savić S, et al. Regional distribution of cytochrome c oxidase activity and copper in sclerotic hippocampi of epilepsy patients. Brain Behav. 2021 Feb;11(2):e01986.
40. Gonzalez‐Lopez E, Vr ana KE. Dopamine beta‐hydroxylase and its genetic variants in human health and disease. J Neurochem. 2020 Jan;152(2):157–81.
41. Aschner M, Skalny AV, Lu R, Martins AC, Tizabi Y, Nekhoroshev SV, et al. Mitochondrial pathways of copper neurotoxicity: focus on mitochondrial dynamics and mitophagy. Front Mol Neurosci. 2024 Dec 5;17:1504802.
42. Peng G, Huang Y, Xie G, Tang J. Exploring Copper's role in stroke: progress and treatment approaches. Front Pharmacol. 2024 Sep 26;15:1409317.
43. Benkirane A, Warlop T, Ivanoiu A, Baret P, Wiame E, Haufroid V, et al. Case report: Motor neuron disease phenotype associated with symptomatic copper deficiency: Challenging diagnosis and treatment. Front Neurol. 2023 Jan 4;13:1063803.
44. Parker SJ, Koistinaho J, White AR, Kanninen KM. Biometals in rare neurodegenerative disorders of childhood. Front Aging Neurosci [Internet]. 2013 [cited 2025 Jul 8];5.
45. Chen Z, Jiang R, Chen M, Zheng J, Chen M, Braidy N, et al. Multi-copper ferroxidase deficiency leads to iron accumulation and oxidative damage in astrocytes and oligodendrocytes. Sci Rep. 2019 Jul 1;9(1):9437.
46. McCarthy RC, Kosman DJ. Ferroportin and Exocytoplasmic Ferroxidase Activity Are Required for Brain Microvascular Endothelial Cell Iron Efflux. J Biol Chem. 2013 Jun;288(24):17932–40.
47. McCarthy RC, Kosman DJ. Glial Cell Ceruloplasmin and Hepcidin Differentially Regulate Iron Efflux from Brain Microvascular Endothelial Cells. Cobine PA, editor. PLoS ONE. 2014 Feb 12;9(2):e89003.
48. Martel G, Hevi C, Friebely O, Baybutt T, Shumyatsky GP. Zinc transporter 3 is involved in learned fear and extinction, but not in innate fear. Learn Mem. 2010 Nov;17(11):582–90.
49. Martel G, Hevi C, Kane-Goldsmith N, Shumyatsky GP. Zinc transporter ZnT3 is involved in memory dependent on the hippocampus and perirhinal cortex. Behav Brain Res. 2011 Sep;223(1):233–8.
50. Elitt CM, Fahrni CJ, Rosenberg PA. Zinc homeostasis and zinc signaling in white matter development and injury. Neurosci Lett. 2019 Aug;707:134247.
51. Beroun A, Mitra S, Michaluk P, Pijet B, Stefaniuk M, Kaczmarek L. MMPs in learning and memory and neuropsychiatric disorders. Cell Mol Life Sci. 2019 Aug;76(16):3207–28.
52. Sensi SL, Paoletti P, Koh JY, Aizenman E, Bush AI, Hershfinkel M. The Neurophysiology and Pathology of Brain Zinc. J Neurosci. 2011 Nov 9;31(45):16076–85.
53. Kimura T, Kambe T. The Functions of Metallothionein and ZIP and ZnT Transporters: An Overview and Perspective. Int J Mol Sci. 2016 Mar 4;17(3):336.
54. Souza PCT, Thallmair S, Marrink SJ, Mera-Adasme R. An Allosteric Pathway in Copper, Zinc Superoxide Dismutase Unravels the Molecular Mechanism of the G93A Amyotrophic Lateral Sclerosis-Linked Mutation. J Phys Chem Lett. 2019 Dec 19;10(24):7740–4.
55. Pitts MW, Byrns CN, Ogawa-Wong AN, Kremer P, Berry MJ. Selenoproteins in Nervous System Development and Function. Biol Trace Elem Res. 2014 Dec;161(3):231–45.
56. Ramos P, Santos A, Pinto NR, Mendes R, Magalhães T, Almeida A. Anatomical Regional Differences in Selenium Levels in the Human Brain. Biol Trace Elem Res. 2015 Feb;163(1–2):89–96.
57. Liang X, Xue Z, Zheng Y, Li S, Zhou L, Cao L, et al. Selenium supplementation enhanced the expression of selenoproteins in hippocampus and played an euro protective role in LPS-induced neuroinflammation. Int J Biol Macromol. 2023 Apr;234:123740.
58. Peters MM, Hill KE, Burk RF, Weeber EJ. Altered hippocampus synaptic function in selenoprotein P deficient mice. Mol Neurodegener [Internet]. 2006 Dec [cited 2025 Jul 10];1(1).
59. Zhang Y, Zhou Y, Schweizer U, Savaskan NE, Hua D, Kipnis J, e t a l. Compa r a tive Ana lysis of Selenocysteine Machinery and Selenoproteome Gene Expression in Mouse Brain Identifies Neurons as Key Functional Sites of Selenium in Mammals. J Biol Chem. 2008 Jan; 283(4):2427–38.
60. Sasuclark AR, Khadka VS, Pitts MW. Cell-Type Specific Analysis of Selenium-Related Genes in Brain. Antioxidants. 2019 May 5;8(5):120.
61. Shahidin, Wang Y, Wu Y, Chen T, Wu X, Yuan W, et al. Selenium and Selenoproteins: Mechanisms, Health Functions, and Emerging Applications. Molecules. 2025 Jan 21;30(3):437.
62. Soerensen J, Jakupoglu C, Beck H, Förster H, Schmidt J, Schmahl W, et al. The Role of Thioredoxin Reductases in Brain Development. Hatfield DL, editor. PLoS ONE. 2008 Mar 19;3(3):e1813.
63. Wu Q, Sun X, Chen Q, Zhang X, Zhu Y. Genetically predicted selenium is negatively associated with serum TC, LDL-C and positively associated with HbA1C levels. J Trace Elem Med Biol. 2021 Sep;67:126785.
64. Sasuclark AR, Watanabe M, Roshto K, Kilonzo VW, Zhang Y, Pitts MW. Selenium deficiency impedes maturation of parvalbumin interneurons, perineuronal nets, and neural network activity. Redox Biol. 2025 Apr;81:103548.
65. Nisar H, Amin R, Khan S, Fatima T, Qamar-Un-Nisa, Jawwad-Us-Salam. Correlation between selenium levels and selenoproteins expression in idiopathic generalized epilepsy: a study from Karachi. BMC Neurol [Internet]. 2025 Jan 23 [cited 2025 Jul 10];25(1).
66. Rita Cardoso B, Silva Bandeira V, Jacob-Filho W, Franciscato Cozzolino SM. Selenium status in elderly: Relation to cognitive decline. J Trace Elem Med Biol. 2014 Oct;28(4):422–6.
67. Liu Y, Liu Y, Shi L, Zhang X, Liu K, He S. Selenium ameliorates cognitive impairment through activating BDNF/TrkB pathway. J Trace Elem Med Biol. 2025 Apr;88:127599.
68. Li Z, Wang W, Xin X, Song X, Zhang D. Association of total zinc, iron, copper and selenium intakes with depression in the US adults. J Affect Disord. 2018 Mar;228:68–74.
69. Marreiro D, Cruz K, Morais J, Beserra J, Severo J, De Oliveira A. Zinc and Oxidative Stress: Current Mechanisms. Antioxidants. 2017 Mar 29;6(2):24.
70. Nosrati R, Kheirouri S, Ghodsi R, Ojaghi H. The effects of zinc treatment on matrix metalloproteinases: A systematic review. J Trace Elem Med Biol. 2019 Dec;56:107–15.
71. Ficiarà E, Munir Z, Boschi S, Caligiuri ME, Guiot C. Alteration of iron concentration in Alzheimer's disease as a possible diagnostic biomarker unveiling ferroptosis. International Journal of Molecular Sciences. 2021 Apr 25;22(9):4479.
72. Duță C, Muscurel C, Dogaru CB, Stoian I. Selenoproteins: Zoom-in to their metal-binding properties in neurodegenerative diseases. Int J Mol Sci. 2025;26(3):1305.
73. Stremmel W, Weiskirchen R. Therapeutic strategies in Wilson disease: pathophysiology and mode of action. Ann Transl Med. 2021;9(8):732.
74. Chen L, Shen Q, Liu Y, Zhang Y, Sun L, Ma X, Song N, Xie J. Homeostasis and metabolism of iron and other metal ions in neurodegenerative diseases. Signal Transduct Target Ther. 2025;10(1):31.
75. Chen HH, Yeo HT, Huang YH, Tsai LK, Lai HJ, Tsao YP, Chen SL. AAV-NRIP gene therapyameliorates motor neuron degeneration and muscle atrophy in ALS model mice. Skelet Muscle.2024;14(1):17.
Downloads
Views | PDF Downloads:
310
/ 146
Published
How to Cite
Issue
Section
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.


